352290-60-9
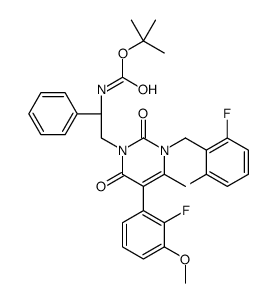
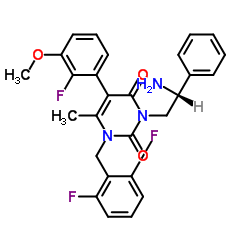
| Name | 3-[(2R)-2-amino-2-phenylethyl]-1-[(2,6-difluorophenyl)methyl]-5-(2-fluoro-3-methoxyphenyl)-6-methylpyrimidine-2,4-dione |
|---|---|
| Synonyms |
3-[(R)-amino-2-phenylethyl]-5-(2-fluoro-3-methoxyphenyl)-1-[2,6-difluorobenzyl]-6-methyl-pyrimidine-2,4(1H,3H)-dione
3-[(2R)-2-Amino-2-phenylethyl]-1-(2,6-difluorobenzyl)-5-(2-fluoro-3-methoxyphenyl)-6-methyl-2,4(1H,3H)-pyrimidinedione 3-[(2R)-2-amino-2-phenylethyl]-1-(2,6-difluorobenzyl)-5-(2-fluoro-3-methoxyphenyl)-6-methylpyrimidine-2,4(1H,3H)-dione 3-[2(R)-amino-2-phenylethyl]-5-(2-fluoro-3-methoxyphenyl)-1-[2,6-difluorobenzyl]-6-methylpyrimidine-2,4(1H,3H)-dione NBI42902 |
| Description | NBI-42902 is an orally active, potent functional and competitive antagonist of GnRH receptor with an IC50 value of 0.79 nM, a Ki value of 0.56 nM, respectively. NBI-42902 inhibits GnRH-stimulated inositol phosphate (IP) accumulation, Ca2+ flux, and ERK1/2 activation. NBI-42902 inhibits serum luteinizing hormone (LH) in castrated male macaques. NBI-42902 can be used for research on sex-hormone-related diseases[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 631.0±65.0 °C at 760 mmHg |
| Molecular Formula | C27H24F3N3O3 |
| Molecular Weight | 495.49 |
| Flash Point | 335.4±34.3 °C |
| Exact Mass | 495.176971 |
| PSA | 79.25000 |
| LogP | 6.42 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.602 |
|
~% 
352290-60-9 |
| Literature: Tucci, Fabio C.; Zhu, Yun-Fei; Struthers, R. Scott; Guo, Zhiqiang; Gross, Timothy D.; Rowbottom, Martin W.; Acevedo, Oscar; Gao, Yinghong; Saunders, John; Xie, Qiu; Reinhart, Greg J.; Liu, Xin-Jun; Ling, Nicholas; Bonneville, Anne K. L.; Chen, Takung; Bozigrian, Haig; Chen, Chen Journal of Medicinal Chemistry, 2005 , vol. 48, # 4 p. 1169 - 1178 |
|
~% 
352290-60-9 |
| Literature: Tucci, Fabio C.; Zhu, Yun-Fei; Struthers, R. Scott; Guo, Zhiqiang; Gross, Timothy D.; Rowbottom, Martin W.; Acevedo, Oscar; Gao, Yinghong; Saunders, John; Xie, Qiu; Reinhart, Greg J.; Liu, Xin-Jun; Ling, Nicholas; Bonneville, Anne K. L.; Chen, Takung; Bozigrian, Haig; Chen, Chen Journal of Medicinal Chemistry, 2005 , vol. 48, # 4 p. 1169 - 1178 |
|
~% 
352290-60-9 |
| Literature: Tucci, Fabio C.; Zhu, Yun-Fei; Struthers, R. Scott; Guo, Zhiqiang; Gross, Timothy D.; Rowbottom, Martin W.; Acevedo, Oscar; Gao, Yinghong; Saunders, John; Xie, Qiu; Reinhart, Greg J.; Liu, Xin-Jun; Ling, Nicholas; Bonneville, Anne K. L.; Chen, Takung; Bozigrian, Haig; Chen, Chen Journal of Medicinal Chemistry, 2005 , vol. 48, # 4 p. 1169 - 1178 |
|
~% 
352290-60-9 |
| Literature: Tucci, Fabio C.; Zhu, Yun-Fei; Struthers, R. Scott; Guo, Zhiqiang; Gross, Timothy D.; Rowbottom, Martin W.; Acevedo, Oscar; Gao, Yinghong; Saunders, John; Xie, Qiu; Reinhart, Greg J.; Liu, Xin-Jun; Ling, Nicholas; Bonneville, Anne K. L.; Chen, Takung; Bozigrian, Haig; Chen, Chen Journal of Medicinal Chemistry, 2005 , vol. 48, # 4 p. 1169 - 1178 |
| Precursor 4 | |
|---|---|
| DownStream 1 | |