504-96-1
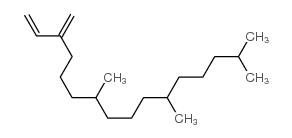
| Name | 7,11,15-trimethyl-3-methylidenehexadec-1-ene |
|---|---|
| Synonyms |
Neophytadien
3-Methylene-7,11,15-trimethylhexadec-1-ene 7,11,15-Trimethyl-3-methylene-1-hexadecene 3-Methylen-7,11,15-trimethyl-hexadecen-(1) 2,6,10-trimethyl-14-ethylene-14-pentadecene neophytadiene 2-(4,8,12-Trimethyltridecyl)buta-1,3-diene |
| Description | Neophytadiene is a diterpene found in Turbinaria ornate, with anti-inflammatory antioxidant and cardioprotective properties[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Neophytadiene has cytotoxic on RAW 264.7 cells with an IC50 of 50 μM[1]. Neophytadiene (50-100 μM; 30 min) successfully inhibits the NO production level and downregulated inducible nitric oxide synthase (iNOS) production in LPS (1 μg/mL)-induced RAW264.7 cells[1]. Neophytadiene (25-100 μM; 22 hours) significantly inhibits the NO production and inflammatory cytokines TNF-α, IL-6 and IL-10 and IL-10 in LPS (1 μg/mL)-induced RAW264.7 cells[1]. |
| In Vivo | Neophytadiene (50 mg/kg; p.o.) shows dose-dependent reduction in the alanine aminotransferase (ALT) level[1]. Neophytadiene (25-50 mg/kg; p.o.) significantly reduces LPS (10 mg/kg)-increased IL6 and 1L10 levels and in heart tissue[1]. Neophytadiene (25-50 mg/kg; p.o.) significantly reduces LPS (10 mg/kg)-increased PGE2mRNA expression in heart tissue[1]. Neophytadiene significantly inhibits LPS (10 mg/kg)-induced NO production and inflammatory cytokines TNF-α, IL-6 and IL-10 and IL-10 in vivo[1]. Animal Model: Adult male Sprague Dawley rats (6-8 weeks)[1] Dosage: 12 mg/kg, 25 mg/kg, 50 mg/kg Administration: Oral administration, daily, for 7 days Result: Decreased haemoglobin (HGB) level, ALT level, and heart tissue TNF-α, IL1β, NF-κB, iNOS, PI3k/AktandMAPK in LPS (10 mg/kg)-treat rats. |
| References |
| Density | 0.796g/cm3 |
|---|---|
| Boiling Point | 344.5ºC at 760mmHg |
| Molecular Formula | C20H38 |
| Molecular Weight | 278.51600 |
| Flash Point | 160.2ºC |
| Exact Mass | 278.29700 |
| LogP | 7.16770 |
| Index of Refraction | 1.449 |
| Storage condition | 20°C |
|
~% 
504-96-1 |
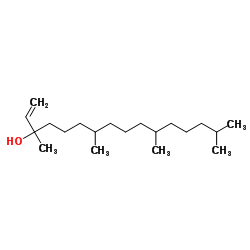
| Literature: Marson, Charles M.; Rioja, Alphonso S.; Brooke, Greg; Coombes; Vigushin, David M. Bioorganic and Medicinal Chemistry Letters, 2002 , vol. 12, # 2 p. 255 - 259 |
|
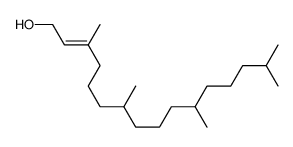
~8% 
504-96-1 |
| Literature: Hyatt, John A.; Kottas, Gregg S.; Effler, Janet Organic Process Research and Development, 2002 , vol. 6, # 6 p. 782 - 787 |
|
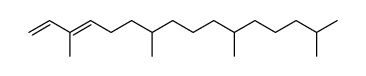
~23% 
504-96-1 |
| Literature: Grossi; Rontani Tetrahedron Letters, 1995 , vol. 36, # 18 p. 3141 - 3144 |
|
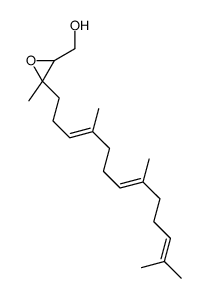
~% 
504-96-1 |
| Literature: Hyatt, John A.; Kottas, Gregg S.; Effler, Janet Organic Process Research and Development, 2002 , vol. 6, # 6 p. 782 - 787 |
|
~% 
504-96-1 |
| Literature: Hyatt, John A.; Kottas, Gregg S.; Effler, Janet Organic Process Research and Development, 2002 , vol. 6, # 6 p. 782 - 787 |
|
~% 
504-96-1 |
| Literature: Hyatt, John A.; Kottas, Gregg S.; Effler, Janet Organic Process Research and Development, 2002 , vol. 6, # 6 p. 782 - 787 |
| Precursor 4 | |
|---|---|
| DownStream 1 | |