910129-15-6
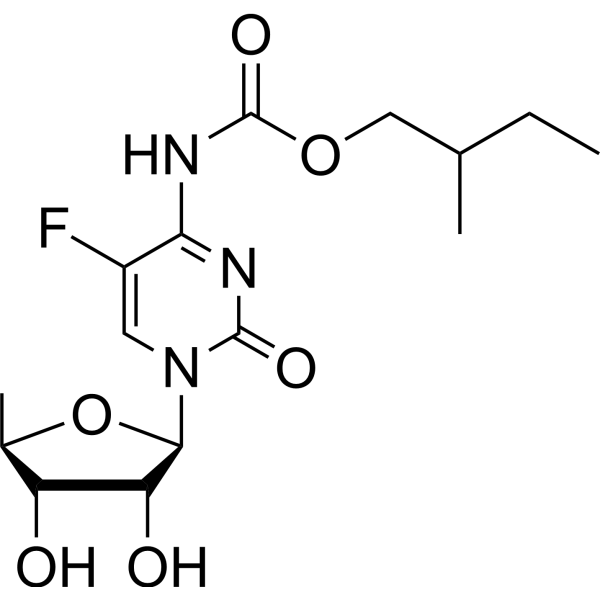
| Name | 2-methylbutyl N-[1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-methyloxolan-2-yl]-5-fluoro-2-oxopyrimidin-4-yl]carbamate |
|---|---|
| Synonyms |
unii-0jd5qg20w5
1-(5-Deoxypentofuranosyl)-5-fluoro-4-{[(pentyloxy)carbonyl]amino}-2(1H)-pyrimidinone 1-(5-deoxypentofuranosyl)-5-fluoro-4-{[(pentyloxy)carbonyl]amino}pyrimidin-2(1h)-one 5'-Deoxy-5-fluoro-N-[(2-methylbutoxy)carbonyl]cytidine Capecitabine Impurity 2 |
| Description | 5′-Deoxy-5-fluoro-N-[(2-methylbutoxy)carbonyl]cytidine is a purine nucleoside analog. Purine nucleoside analogs have broad antitumor activity targeting indolent lymphoid malignancies. Anticancer mechanisms in this process rely on inhibition of DNA synthesis, induction of apoptosis, etc[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Melting Point | 62-64°C |
| Molecular Formula | C15H22FN3O6 |
| Molecular Weight | 359.35 |
| Exact Mass | 359.149261 |
| PSA | 126.40000 |
| LogP | 0.97 |
| Index of Refraction | 1.600 |
| Storage condition | Hygroscopic, -20°C Freezer, Under Inert Atmosphere |
| Hazard Codes | Xn |
|---|