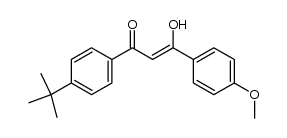
70356-09-1
| Name | 1-(4-tert-Butylphenyl)-3-(4-methoxyphenyl)-1,3-propanedione |
|---|---|
| Synonyms |
1-[4-(1,1-Dimethylethyl)phenyl]-3-(4-methoxyphenyl)-1,3-propanedione
MFCD00210252 1-(4-tert-Butylphenyl)-3-(4-methoxyphenyl)propane-1,3-dione 4-tert-Butyl-4'-methoxydibenzoylmethane Avobenzone 1-(4-Methoxyphenyl)-3-[4-(2-methyl-2-propanyl)phenyl]-1,3-propanedione 1X1&1&R DV1VR DO1 EINECS 274-581-6 Avobenzone (USP) |
| Description | Avobenzone is an oil soluble ingredient used in sunscreen products to absorb the full spectrum of UVA rays and a dibenzoylmethane derivative.Target: OthersAvobenzone is an oil soluble ingredient used in sunscreen products to absorb the full spectrum of UVA rays and a dibenzoylmethane derivative. It can degrade faster in light in combination with mineral UV absorbers like zinc oxide and titanium dioxide, though with the right coating of the mineral particles this reaction can be reduced. A manganese doped titanium dioxide may be better than undoped titanium dioxide to improve avobenzone's stability. It reacts with minerals to form colored complexes. Manufacturers of avobenzone, like DSM recommend to include a chelator to prevent this from happening. They also recommend to avoid the inclusion of iron and ferric salts, heavy metals, formaldehyde donors and PABA and PABA esters[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 463.6±35.0 °C at 760 mmHg |
| Melting Point | 81-84 °C |
| Molecular Formula | C20H22O3 |
| Molecular Weight | 310.387 |
| Flash Point | 203.1±26.0 °C |
| Exact Mass | 310.156891 |
| PSA | 43.37000 |
| LogP | 4.81 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.545 |
| Symbol |

GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H410 |
| Precautionary Statements | P273-P501 |
| Personal Protective Equipment | Eyeshields;Gloves |
| Hazard Codes | N |
| Risk Phrases | R50/53 |
| Safety Phrases | S60-S61 |
| RIDADR | UN3077 9/PG 3 |
| HS Code | 29145000 |
|
~96% 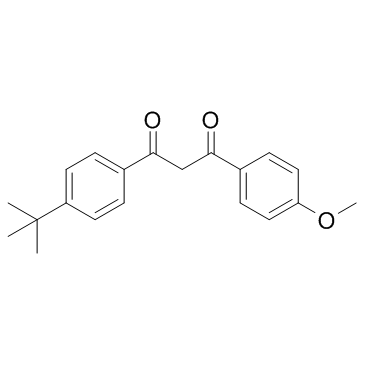
70356-09-1 |
| Literature: DSM IP ASSETS B.V.; WEHRLI, Christof Patent: WO2012/84770 A1, 2012 ; Location in patent: Page/Page column 8-9 ; |
|
~93% 
70356-09-1 |
| Literature: DSM IP ASSETS B.V.; WEHRLI, Christof Patent: WO2012/84770 A1, 2012 ; Location in patent: Page/Page column 10-11 ; |
|
~% 
70356-09-1 |
| Literature: US2013/58879 A1, ; Paragraph 0194 ; |
|
~% 
70356-09-1 |
| Literature: Journal de Chimie Physique et de Physico-Chimie Biologique, , vol. 95, # 2 p. 388 - 394 |
|
~% 
70356-09-1 |
| Literature: Journal of Photochemistry and Photobiology A: Chemistry, , vol. 209, # 2-3 p. 153 - 157 |
|
~% 
70356-09-1 |
| Literature: RSC Advances, , vol. 3, # 43 p. 19785 - 19788 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |
| HS Code | 2909499000 |
|---|---|
| Summary | 2909499000. ether-alcohols and their halogenated, sulphonated, nitrated or nitrosated derivatives. VAT:17.0%. Tax rebate rate:9.0%. . MFN tariff:5.5%. General tariff:30.0% |