111841-85-1
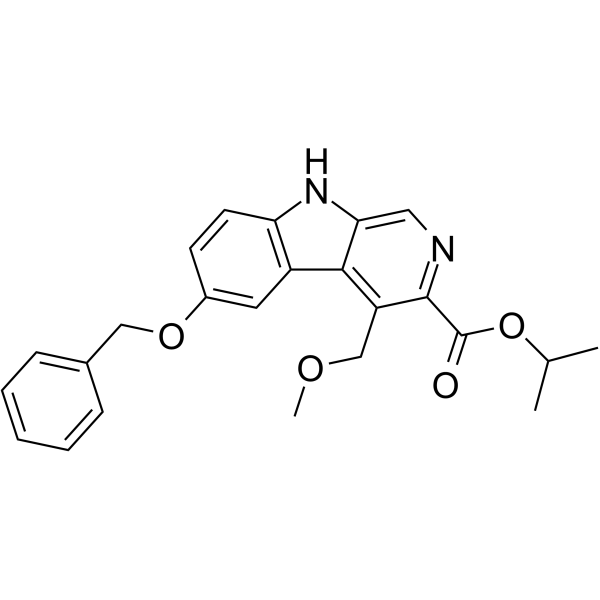
| Name | propan-2-yl 4-(methoxymethyl)-6-phenylmethoxy-9H-pyrido[3,4-b]indole-3-carboxylate |
|---|---|
| Synonyms |
9H-Pyrido(3,4-b)indole-3-carboxylic acid,1-(methoxymethyl)-6-(phenylmethoxy)-,1-methylethyl ester
6-benzyloxy-4-methoxymethyl-9H-pyrido[3,4-b]indole-3-carboxylic acid-1-methylethyl-ester Abecarnil isopropyl 6-benzyloxy-4-methoxymethyl-9H-pyrido[3,4-b]indole-3-carboxylate Abecarnil (INN) Abecarnilum [INN-Latin] Propan-2-Yl 4-(Methoxymethyl)-6-(Phenylmethoxy)-9H-Pyrido[5,4-b]Indole-3-Carboxylate |
| Description | Abecarnil (ZK 112119) is a ligand or a partial agonist for benzodiazepine (BZ) receptor. Abecarnil possesses anxiolytic and anticonvulsant properties. Abecarnil can act as a positive allosteric modulator of GABAA receptor. Abecarnil inhibits the binding of the BZ [3H]lormetazepam to rat cerebral cortex membranes, with an IC50 of 0.82 nM. Abecarnil can be used for epilepsy research[1][2][3][4]. |
|---|---|
| Related Catalog | |
| In Vitro | Abecarnil enhances the binding of t-[35S]butylbicyclophosphorothionate to rat cortical membranes[1]. Abecarnil exhibits a 3- to 6-fold higher affinity to forebrain BZ receptors than Diazepam (DZP)[1]. |
| In Vivo | Abecarnil (0.3 mg/kg, IP, once) antagonizes the brain neuroactive steroid increase induced by foot shock[2]. Abecarnil (0-2.5 mg/kg, IP, once) dose dependently reduces epileptic activity[3]. Abecarnil is effective against sound-induced convulsions in DBA/2 mice, against air blast-induced generalized seizures in gerbils and against myoclonus in baboons Papio papio[4]. Abecarnil is 2-10 times more potent than DZP in most rodent tests of anxiolytic activity, and in reducing locomotor activity in mice and rats thoroughly habituated to the test chamber[1]. Animal Model: Male Sprague-Dawley CD rats (200-250 g)[2] Dosage: 0.3 mg/kg Administration: IP, once, given 30 min before sacrifice Result: Failed to change the basal pregnenolone and progesterone, while only slightly decreased THDOC levels, but antagonized the brain neuroactive steroid increase induced by foot shock. Animal Model: WAG/Rij rats (male and female, 190-380 g, age 13-19 weeks, 8 rats each group)[3] Dosage: 0, 0.16, 0.4, 1.0, and 2.5 mg/kg; 1 mL/400 g Administration: IP, once Result: Reduced the duration of spike-wave discharges and increased immobile behavior. Dose dependently reduced epileptic activity, whether measured as number, mean duration, or total duration of spike-wave discharges. The ED50 for reducing the number of spike-wave discharges in the second hour was 0.4 mg/kg. |
| References |
| Density | 1.246g/cm3 |
|---|---|
| Boiling Point | 620.2ºC at 760mmHg |
| Molecular Formula | C24H24N2O4 |
| Molecular Weight | 404.45800 |
| Flash Point | 328.9ºC |
| Exact Mass | 404.17400 |
| PSA | 73.44000 |
| LogP | 5.00670 |
| Vapour Pressure | 2.62E-15mmHg at 25°C |
| Index of Refraction | 1.647 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xn |
|---|