70052-12-9
| Name | Eflornithine |
|---|---|
| Synonyms |
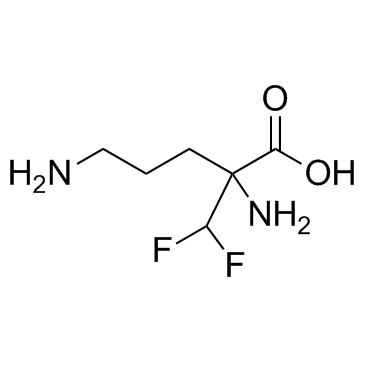
2-(difluoromethyl)-2,5-diaminopentanoic acid
2-(2-ethyl-2,3-dihydro-2-benzofuranyl)-4,5-dihydro-1H-imidazole hydrochloride MFCD00242734 2,5-diamino-2-(difluoromethyl)pentanoic acid 2-Ethyl-2-(imidazolin-2-yl)-2,3-dihydrobenzofuran hydrochloride Efaroxan HCl H-DL-(a-difluoromethyl)Orn-OH Efaroxan hydrochloride |
| Description | Eflornithine is a specific, irreversible inhibitor of the enzyme ornithine decarboxylase. Eflornithine is a medication for the treatment of African trypanosomiasis and excessive facial hair growth in women. |
|---|---|
| Related Catalog | |
| In Vivo | Eflornithine is the only new molecule registered for the treatment of human African trypanosomiasis over the last 50 years. It is the drug used mainly as a back-up for melarsoprol refractory Trypanosoma brucei gambiense cases[1]. In subjects with excessive, unwanted facial hair, eflornithine 15% cream is superior to placebo in reducing hair growth. After 24 weeks' treatment, 58% of eflornithine and 34% of placebo subjects have at least some improvement in facial hirsutism[2]. The hair growth inhibitory activity of eflornithine is significantly enhanced when the eflornithine cream is applied onto a mouse skin area pretreated with microneedles[3]. Treatment of coarctation hypertensive rats with eflornithine results in a normalization of the contractile intensity to KCI and norepinephrine and relaxations to acetylcholine by 14 days of hypertension[4]. |
| Animal Admin | Mice: The skin area where the hair is removed is then treated with the eflornithine hydrochloride 13.9% cream (∼50 mg per mouse per treatment) using a spatula 2 times a day in an interval of at least 8 h for a maximum period of 36 days[3]. |
| References |
[2]. Balfour JA, et al. Topical eflornithine. Am J Clin Dermatol. 2001;2(3):197-201; discussion 202. |
| Density | 1.293g/cm3 |
|---|---|
| Boiling Point | 347ºC at 760 mmHg |
| Molecular Formula | C6H12F2N2O2 |
| Molecular Weight | 182.16800 |
| Flash Point | 163.7ºC |
| Exact Mass | 182.08700 |
| PSA | 89.34000 |
| LogP | 1.17310 |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Precursor 1 | |
|---|---|
| DownStream 0 | |