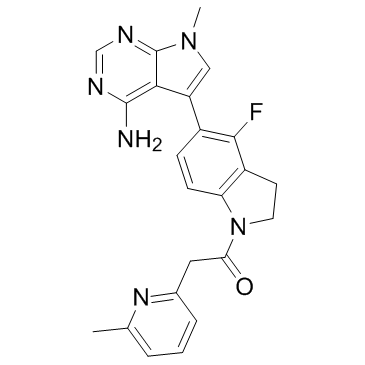
1337532-29-2
| Name | 1-(5-(4-amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-4-fluoroindolin-1-yl)-2-(6-methylpyridin-2-yl)ethan-1-one |
|---|---|
| Synonyms |
1-[5-(4-Amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-4-fluoro-2,3-dihydro-1H-indol-1-yl]-2-(6-methyl-2-pyridinyl)ethanone
GSK2656157 |
| Description | GSK2656157 is a selective and ATP-competitive inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) with an IC50 of 0.9 nM. |
|---|---|
| Related Catalog | |
| Target |
EIF2AK3 (PERK):0.9 nM (IC50) EIF2AK1 (HRI):460 nM (IC50) BRK:905 nM (IC50) EIF2AK2 (PKR):905 nM (IC50) MEKK3:954 nM (IC50) Aurora B:1259 nM (IC50) KHS:1764 nM (IC50) LCK:2344 nM (IC50) MLK2:2796 nM (IC50) MEKK3:2847 nM (IC50) ALK5:3020 nM (IC50) MLCK2:3039 nM (IC50) EIF2AK4(GCN2):3162 nM (IC50) c-MER:3431 nM (IC50) PI3Kγ:3802 nM (IC50) WNK3:5951 nM (IC50) LRRK2:6918 nM (IC50) ROCK1:7244 nM (IC50) MSK1:8985 nM (IC50) NEK1:9807 nM (IC50) AXL:9808 nM (IC50) JAK2:24547 nM (IC50) |
| In Vitro | GSK2656157 results in inhibition of PERK activation as well as decreases in the downstream substrates, phospho-eIF2α, ATF4, and CHOP with an IC50 in the range of 10-30 nM in the BxPC3 pancreatic tumor cell line. Cells that are exposed to 1 μM GSK2656157 before UPR induction are able to block this effect on de novo protein synthesis[1]. GSK2656157 causes the activation of another eIF2α kinase to compensate for the loss of PERK activity in HT1080 cells. GSK2656157 inhibits the growth of the HT1080 cells[2]. GSK2656157 inhibits LPS-induced IL-1β production, LPS-induced Caspase 1 activation and LPS-induced eIF-2α phosphorylation, but does not inhibit LPS-induced TNF-α production[3]. |
| In Vivo | GSK2656157 (1.5-150 mg/kg, p.o.) results in dose-dependent inhibition of phospho-PERK Thr980, with more than 80% inhibition at 50 and 150 mg/kg. GSK2656157 (50-150 mg/kg, p.o.) results in dose-dependent inhibition of tumor growth in human tumor xenograft models[1]. |
| Kinase Assay | BxPC3 (human pancreatic adenocarcinoma) or LL/2 (murine lung carcinoma) cells are treated with DMSO or various concentrations of GSK2656157 for 1 hour, followed by addition of 5 μg/mL tunicamycin or 1 μM thapsigargin for an additional 6 hours to induce endoplasmic reticulum-stress. Cells are lysed in cold radioimmunoprecipitation assay (RIPA) buffer [150 mM NaCl, 50 mM Tris-Cl pH 7.5, 0.25% sodium deoxycholate, 1% NP-40, protease inhibitors, and 100 mM sodium orthovanadate]. Clarified lysates are resolved by SDS-PAGE and transferred to nitrocellulose membrane using NuPAGE system. Blots are incubated with antibodies to total PERK, p-eIF-2α Ser51, total eIF-2α, ATF4, and CHOP. IRDye700DX-labeled goat anti-mouse immunoglobulin G (IgG), IRDye800-CW donkey anti-goat IgG, and IRDye800-CW goat anti-rabbit IgG are used as secondary antibodies. Proteins are detected on the Odyssey Infrared Imager. |
| Cell Assay | BxPC3 cells are treated with DMSO or 1 μM GSK2656157 for 1 hour before adding 5 μg/mL tunicamycin for an additional hour. Cells are metabolically labeled with 125 μCi 35S-methionine for the subsequent 1 hour. Cells are lysed in cold RIPA buffer and lysates are resolved by SDS-PAGE, followed by exposure to a phosphorimager screen. Control cells are also pretreated with 100 μM cyclohexamide for 1 hour followed by metabolic labeling. Radioisotope incorporation is quantitated using ImageQuant 5.2 software. |
| Animal Admin | Exponentially growing HPAC (5×106 cells/mouse), Capan-2 (10×106 cells/mouse), or NCI-H929 (1×6 cells/mouse) cells are implanted subcutaneously into the right flank of 8- to 12-week-old female SCID mice. Similarly, 10×106 BxPC3 cells per mouse are implanted in female nude mice. When the tumors reached approximately 200 mm3 in size, the animals are weighed, and block randomized according to tumor size into treatment groups of 8 mice each. Mice are dosed orally with the formulating vehicle or GSK2656157. Mice are weighed and tumors measured by calipers twice weekly. Tumor volumes are calculated. The percentage of tumor growth inhibition is calculated on each day of tumor measurement using the formula: 100× [1 − (average growth of the compound-treated tumors/average growth of vehicle-treated control tumors)]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 744.6±60.0 °C at 760 mmHg |
| Molecular Formula | C23H21FN6O |
| Molecular Weight | 416.451 |
| Flash Point | 404.1±32.9 °C |
| Exact Mass | 416.176086 |
| PSA | 89.93000 |
| LogP | 3.43 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.722 |
| Storage condition | -20℃ |