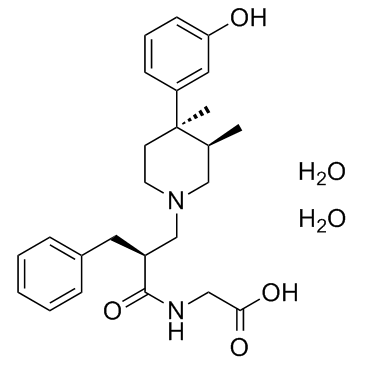
170098-38-1
| Name | 2-[[(2S)-2-benzyl-3-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]propanoyl]amino]acetic acid,dihydrate |
|---|---|
| Synonyms |
Alvimopan dihydrate
Alvimopan N-{(2S)-2-Benzyl-3-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]propanoyl}glycine dihydrate Glycine, N-[(2S)-3-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]-1-oxo-2-(phenylmethyl)propyl]-, hydrate (1:2) Alvimopan (USAN) Adl 8-2698 Alvimopan anhydrous UNII-677C126AET Alvimopan hydrate Entereg Alvimopan (dihydrate) |
| Description | Alvimopan dihydrate(LY 246736; ADL 8-2698) is a peripherally acting mu-opioid receptor (PAM-OR, IC50= 1.7 nM) antagonist for accelerating gastrointestinal recovery after surgery. IC50 Value: 1.7 nM (Mu-type opioid receptor) [1]Target: mu-opioid receptorin vitro: The dissociation rate of alvimopan from the micro opioid receptor (t(1/2)=30--44 min) was comparable to that of the long acting partial agonist buprenorphine (t(1/2)=44 min), but was slower than those of the antagonists naloxone (t(1/2)=0.82 min) and N-methylnaltrexone (t(1/2)=0.46 min) [2].in vivo: Alvimopan did not significantly accelerate GI-3 compared with placebo [6 mg: hazard ratio (HR) = 1.20, p = 0.080; 12 mg: HR = 1.24, p = 0.038). However, after adjustment for significant covariates (sex/surgical duration), benefits were significant for both doses (6 mg: HR = 1.24, p = 0.037; 12 mg: HR = 1.26, p = 0.028). Alvimopan also significantly accelerated time to GI-2 (6 mg: HR = 1.37, p = 0.008; 12 mg: HR = 1.33, p = 0.018) and DCO (6 mg: HR = 1.31, p = 0.008; 12 mg: HR = 1.28, p = 0.015) [3]. Alvimopan (1 and 3 mg/kg) significantly reversed this delayed GI transit when administered 45 min prior to surgery. However, the effects of alvimopan were less pronounced when administered following surgery [4].Toxicity:The most common treatment-emergent adverse events across all treatment groups were nausea, vomiting, and hypotension; the incidence of nausea and vomiting was reduced by 53 percent in thealvimopan 12-mg group [5].Clinical trial: Intercostal Nerve Block With Liposome Bupivacaine in Subjects Undergoing Posterolateral Thoracotomy. Phase 3 |
|---|---|
| Related Catalog | |
| References |
| Density | 1.166g/cm3 |
|---|---|
| Boiling Point | 684.1ºC at 760mmHg |
| Melting Point | 210-213ºC |
| Molecular Formula | C25H36N2O6 |
| Molecular Weight | 460.563 |
| Flash Point | 367.5ºC |
| Exact Mass | 460.257324 |
| PSA | 108.33000 |
| LogP | 3.25160 |
| Vapour Pressure | 1.18E-19mmHg at 25°C |
| Index of Refraction | 1.572 |
| Storage condition | -20℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|