57186-25-1
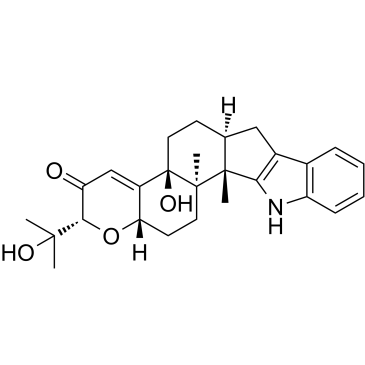
| Name | Paxilline,(2R,4bS,6aS,12bS,12cR,14aS)-5,6,6a,7,12,12b,12c,13,14,14a-Decahydro-4b-hydroxy-2-(1-hydroxy-1-methylethyl)-12b,12c-dimethyl-2H-pyrano[2'',3'':5',6']benz[1',2':6,7]indeno[1,2-b]indol-3(4bH)-one |
|---|---|
| Synonyms |
PAXILINE
PAXILLINE,PENICILLIUM PAXILLI DPNI-caged-GABA (2R,4bS,6aS,12bS,12cR,14aS)-4b-Hydroxy-2-(2-hydroxy-2-propanyl)-12b,12c-dimethyl-5,6,6a,7,12,12b,12c,13,14,14a-decahydro-2H-chromeno[5',6':6,7]indeno[1,2-b]indol-3(4bH)-one MFCD00083464 (2R,4bS,6aS,12bS,12cR,14aS)-4b-Hydroxy-2-(2-hydroxypropan-2-yl)-12b,12c-dimethyl-5,6,6a,7,12,12b,12c,13,14,14a-decahydro-2H-chromeno[5',6':6,7]indeno[1,2-b]indol-3(4bH)-one paxilline from penicillium paxilli Paxilline 1 Paxilline |
| Description | Paxilline is an indole alkaloid mycotoxin from Penicillium paxilli, acts as a potent BK channels inhibitor by an almost exclusively closed-channel block mechanism. Paxilline also inhibits the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) with IC50s between 5μM and 50μM for differing isoforms. Paxilline possesses significant anticonvulsant activity[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 5-50 μM (SERCA)[2], BK channel[1] |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 648.8±55.0 °C at 760 mmHg |
| Melting Point | 252ºC |
| Molecular Formula | C27H33NO4 |
| Molecular Weight | 435.555 |
| Flash Point | 346.2±31.5 °C |
| Exact Mass | 435.240967 |
| PSA | 82.55000 |
| LogP | 3.77 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.661 |
| Storage condition | 2-8°C |
| Water Solubility | Soluble in DMSO, acetone or chloroform. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS05, GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H311-H315-H318-H331-H335 |
| Precautionary Statements | P261-P280-P301 + P310-P305 + P351 + P338-P311 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R23/24/25 |
| Safety Phrases | 26-36/37/39-45 |
| RIDADR | UN 2811 6 |
| WGK Germany | 3 |
| RTECS | DJ2830000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 29419090 |
| Precursor 0 | |
|---|---|
| DownStream 2 | |