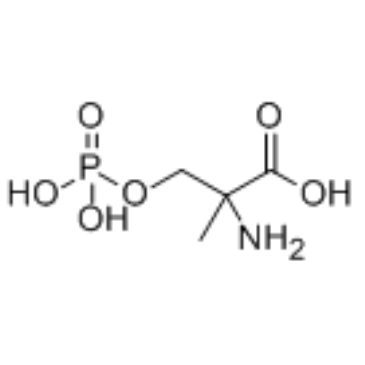
66515-29-5
| Name | 2-amino-2-methyl-3-phosphonooxypropanoic acid |
|---|---|
| Synonyms |
MSOP
Cyprodime hydrochloride 2-methyl-O-phosphonoserine |
| Description | MSOP is a selective group III metabotropic glutamate receptor antagonist with apparent KD of 51 μM for the L-AP4-sensitive presynaptic mGluR. |
|---|---|
| Related Catalog | |
| Target |
KD: 51 μM (L-AP4-sensitive presynaptic mGluR)[1]. |
| In Vitro | In the presence of 200 μM MSOP, a rightward parallel shift of the dose-response curve to L-AP4 is observed, with an apparent KD calculated as 51±6 μM (n=3). MSOP is shown to be selective for the L-APC sensitive presynaptic mGluR, the apparent KD for the interaction of MSOP with the (1S, 3S)-ACPD sensitive receptor calculated as greater than 700 μM (n=3)[1]. |
| In Vivo | It is found that TBOA-induced antinociceptive effects are significantly blocked by intrathecal co-administration of MSOP (second phase of formalin model: F3,16=30.96, P<0.001; CFA model: F3,16=30.77, P<0.001). As expected, intrathecal TBOA (10 μg) reduces the number of formalin-induced flinches and shakes by 47% of the value in the saline-treated group in the second phase (P<0.001) and blocked the CFA-induced decrease in ipsilateral paw withdrawal latency by 60% of the value in the saline-treated group (P=0.01). The number of formalin-induced flinches in the second phase in the group treated with MSOP and TBOA is increased by 56% (P=0.04) of the value in the TBOA-treated group. CFA-induced paw withdrawal latency in the group treated with MSOP and TBOA is decreased by 86% (P=0.03) of the value in the TBOA-treated group[2]. |
| Animal Admin | Rats[2] Male Sprague-Dawley rats (250-300 g) are housed individually in cages on a standard 12 h-12 h light-dark cycle. Water and food are available as libitum until rats are transported to the labotatory approximately 1 h before the experiments. A glutamate transporter activator, three glutamate transporter inhibitors, TBOA, DL-THA, dihydrokainate, and a selective group III mGluR antagonist MSOP are used. All drugs are dissolved in 0.9% physiological saline. To examine the role of group III mGluRs in the antinociceptive effect produced by intrathecal TBOA in the formalin model, the rats are intrathecally injected with saline (10 μL; n=5), MSOP (10 μg/10 μL; n=5), TBOA (10 μg/10 μL; n=5), or MSOP plus TBOA (n=5). Ten minutes later, 2% formalin (100 μL) is injected into the plantar side of a hind paw and formalin-induced pain behaviors are assessed.To examine the role of group III mGluRs in the antinociceptive effect produced by intrathecal TBOA in the complete Freund’s adjuvant (CFA) model, the rats are intrathecally injected with saline (10 μL; n=5), MSOP (10 μg/10 μL; n=5), TBOA (10 μg/10 μL; n=5), or MSOP plus TBOA (n=5) at 6 h post-CFA and then measured paw withdrawal latencies[2]. |
| References |
| Molecular Formula | C4H10NO6P |
|---|---|
| Molecular Weight | 199.10 |
| Exact Mass | 199.02500 |
| PSA | 139.89000 |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| RIDADR | UN 2811 6.1 / PGIII |