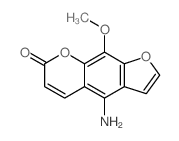
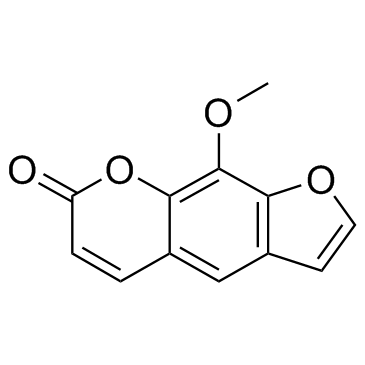
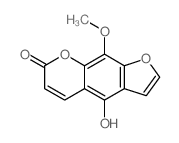
7471-73-0
| Name | 4-hydroxy-9-methoxyfuro[3,2-g]chromen-7-one |
|---|---|
| Synonyms |
4-Hydroxy-9-methoxy-furo[3,2-g]chromen-7-on
xanthotoxin 4-hydroxy-9-methoxy-7H-furo<3,2-g><1>benzopyran-7-one 4-hydroxy-9-methoxy-furo[3,2-g]chromen-7-one 5-hydroxy-8-methoxypsoralen 5-Hydroxyxanthotoxin 8-methoxy-5-hydroxypsoralen |
| Description | 5-Hydroxy-8-methoxypsoralen (5-Hydroxyxanthotoxin) is a metabolite of Xanthotoxin. Xanthotoxin is a potent tricyclic furocoumarin suicide inhibitor of CYP (cytochrome P-450), is an agent used to treat psoriasis, eczema, vitiligo and some cutaneous Lymphomas in conjunction with exposing the skin to sunlight[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.484g/cm3 |
|---|---|
| Boiling Point | 342.7ºC at 760 mmHg |
| Molecular Formula | C12H8O5 |
| Molecular Weight | 232.18900 |
| Flash Point | 161.1ºC |
| Exact Mass | 232.03700 |
| PSA | 72.81000 |
| LogP | 2.25340 |
| Index of Refraction | 1.67 |
|
~% 
7471-73-0 |
| Literature: Brokke; Christensen Journal of Organic Chemistry, 1958 , vol. 23, p. 589,594 |
|
~% 
7471-73-0 |
| Literature: Brokke; Christensen Journal of Organic Chemistry, 1958 , vol. 23, p. 589,594 |
|
~% 
7471-73-0 |
| Literature: Brokke; Christensen Journal of Organic Chemistry, 1958 , vol. 23, p. 589,594 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |