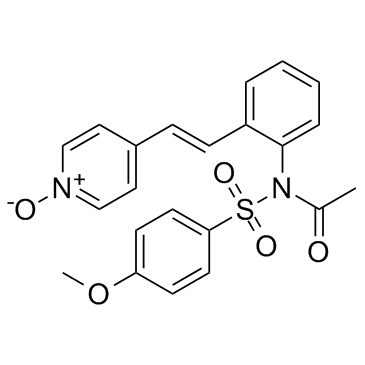
173529-46-9
| Name | N-(4-methoxyphenyl)sulfonyl-N-[2-[(E)-2-(1-oxidopyridin-1-ium-4-yl)ethenyl]phenyl]acetamide |
|---|---|
| Synonyms |
N-[(4-Methoxyphenyl)sulfonyl]-N-{2-[(E)-2-(1-oxido-4-pyridinyl)vinyl]phenyl}acetamide
IVX-214 HMN-214 (E)-4-(2-(2-(N-Acetyl-N-((p-methoxyphenyl)sulfonyl)amino)phenyl)ethenyl)pyridine 1-oxide cc-31 Acetamide,N-((4-methoxyphenyl)sulfonyl)-N-(2-((1E)-2-(1-oxido-4-pyridinyl)ethenyl)phenyl) Acetamide,N-((4-methoxyphenyl)sulfonyl)-N-(2-(2-(1-oxido-4-pyridinyl)ethenyl)phenyl)-,(E) |
| Description | HMN-214, an orally bioavailable prodrug of HMN-176, is an inhibitor of polo-like kinase-1 (plk1), with antitumor activity. |
|---|---|
| Related Catalog | |
| Target |
PLK1 |
| In Vitro | HMN-214 is a prodrug of HMN-176. HMN-176 shows potent activities against 22 human tumor cell lines, with a mean IC50s of 118 nM[1]. HMN-176 (3-300 nM) inhibits luciferase expression driven by the MDR1 promoter in a dose dependent manner in HeLa cells. HMN-176 (30-3000 nM) also dose-dependently suppresses complex formation on the Y-box[3]. HMN-214 (3.3 μM) enhances luciferase expression relative to vehicle control with the 1,4C-1,4Bis polymer (11-fold) and PEI (37-fold) in PC3-PSMA cells. HMN-214 (≥ 3.3 μM) significantly reduces cell proliferation, causes considerable changes in cell morphology in MB49 cells[4]. |
| In Vivo | HMN-214 (33 mg/kg, p.o.) converts to HMN-176 in rats. HMN-214 has no effect on the conduction velocity and the amplitude of action potentials in the aciatic and tibial nerves. HMN-214 (20 mg/kg, p.o.) exhibits antitumor activity in mice[1]. HMN-214 (10, 20 mg/kg, p.o.) decreases MDR1 mRNA expression in nude mice bearing KB- and KB-A.1.-derived tumors[3]. |
| Cell Assay | Cell proliferation in case of different treatment conditions, relative to untreated control cells (treated as 100% viable, or a live control), is quantified using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), a yellow colored reagent which is converted to formazan (a purple dye) by living cells. For screening experiments, transfections are carried out in 96-well cell culture plates, that are seeded with 50,000 cells per well. Following 48 h of transfection, 10 μL of MTT reagent is added to the cells and incubated at 37°C for 2-4 h, and the cells are then lysed by adding 20 μL of MTT detergent and incubated for an additional 2 h at room temperature. Inhibitor dose-optimization transfections are carried out in 24-well plates that are seeded with 50,000 cells per well. After 48 h, 20 μL MTT reagent is added, followed by 100 μL of MTT detergent for lysis for 2 h[4]. |
| Animal Admin | The ground HMN-214 is suspended with an agate pestle by gradually adding 0.5% methylcellulose 4000 solution to make a 3 mg/mL suspension. This is additionally diluted with methylcellulose 4000 solution to obtain suspensions of the appropriate concentration. Tumor tissue is grown in advance by s.c. transplantation into nude mice. The resulting tumors are removed, cut into cubic fragments of 8 mm3, and transplanted s.c. into the right axillary region of nude mice with a trocar. When the theoretical volume of the tumor had reached about 145 mm3, oral administration of HMN-214 is started (day 1)[3]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 663.1±65.0 °C at 760 mmHg |
| Molecular Formula | C22H20N2O5S |
| Molecular Weight | 424.470 |
| Flash Point | 354.8±34.3 °C |
| Exact Mass | 424.109283 |
| PSA | 97.52000 |
| LogP | 1.85 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.598 |
| Storage condition | -20°C |