773059-23-7
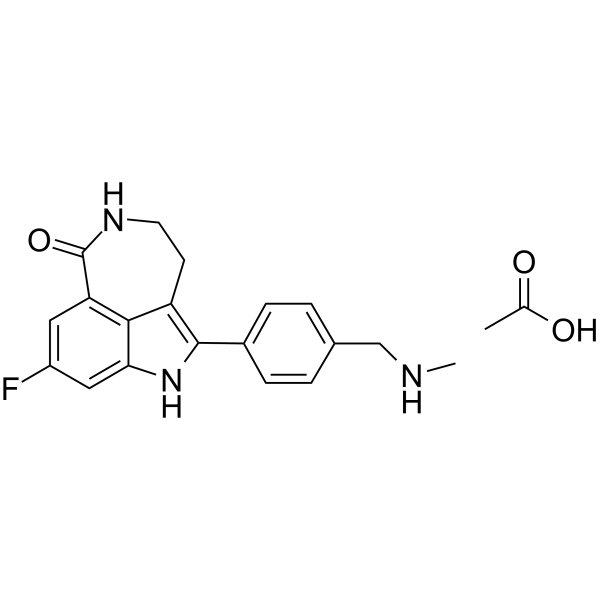
| Name | 8-fluoro-2-(4-methylaminomethyl-phenyl)-1,3,4,5-tetrahydro-azepino[5,4,3-cd]indol-6-one acetate |
|---|---|
| Synonyms | 8-fluoro-1,3,4,5-tetrahydro-2-[4-[(methylamino)methyl]phenyl]-6H-Pyrrolo[4,3,2-ef][2]benzazepin-6-oneacetate |
| Description | Rucaparib (AG014699) acetate is an orally active, potent inhibitor of PARP proteins (PARP-1, PARP-2 and PARP-3) with a Ki of 1.4 nM for PARP1. Rucaparib acetate is a modest hexose-6-phosphate dehydrogenase (H6PD) inhibitor. Rucaparib acetate has the potential for castration-resistant prostate cancer (CRPC) research[1][2][3][4]. |
|---|---|
| Related Catalog | |
| Target |
PARP-1:1.4 nM (Ki) PARP-2 PARP-3 |
| In Vitro | Rucaparib (AG014699) acetate is a possible N-demethylation metabolite of AG14644[1]. Rucaparib (0.1, 1, 10, 100 μM; 24 hours) acetate is cytotoxic and has the LC50 being 5 μM in Capan-1 (BRCA2 mutant) cells and only 100 nM in MX-1 (BRCA1 mutant) cells[2]. The radio-sensitization by Rucaparib acetate is due to downstream inhibition of activation of NF-κB, and is independent of SSB repair inhibition. Rucaparib acetate can target NF-κB activated by DNA damage and overcome toxicity observed with classical NF-κB inhibitors without compromising other vital inflammatory functions[5]. Rucaparib acetate inhibits PARP-1 activity by 97.1% at a concentration of 1 μM in permeabilised D283Med cells[6]. |
| In Vivo | Rucaparib (AG014699) acetate and AG14584 significantly increase Temozolomide toxicity. Rucaparib (1 mg/kg) acetate significantly increases Temozolomide-induced body weight loss. Rucaparib (0.1 mg/kg) acetate esults in a 50% increase in the temozolomide-induced tumor growth delay[1]. Rucaparib (10 mg/kg for i.p. or 50, 150 mg/kg for p.o.; daily for 5 days per week for 6 weeks) acetate significantly inhibits the growth of the tumor, and there is one complete tumor regression and two persistent partial regressions[2]. Rucaparib (150 mg/kg; p.o.; once per week for 6 weeks or three times per week for 6 weeks) acetate has greatest antitumor effect with three complete regressions[2]. Rucaparib acetate enhances the antitumor activity of temozolomide and indicates complete and sustained tumor regression in NB1691 and SHSY5Y xenografts[6]. |
| References |
[3]. Matt Shirley, et al. Rucaparib: A Review in Ovarian Cancer. Target Oncol. 2019 Apr;14(2):237-246. |
| Molecular Formula | C21H22FN3O3 |
|---|---|
| Molecular Weight | 383.41600 |
| Exact Mass | 383.16500 |
| PSA | 94.22000 |
| LogP | 3.78990 |