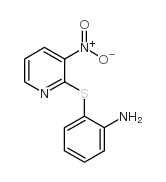
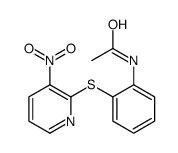
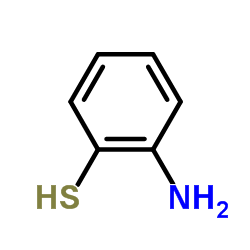
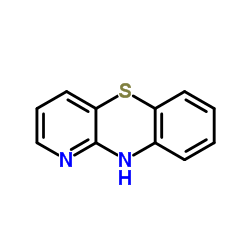
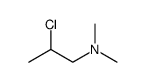
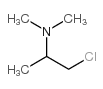
482-15-5
| Name | N,N-dimethyl-1-pyrido[3,2-b][1,4]benzothiazin-10-ylpropan-2-amine |
|---|---|
| Synonyms |
Actapront
Odantol Nilergex Isothipendylum isothiopendyl Isothipendyl Udantol Andanton |
| Description | Isothipendyl (AY 56012), an azaphenothiazine derivative, is a potent histamine 1 (H1) receptor antagonist. Isothipendyl is a primary metabolite[1]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| Density | 1.167g/cm3 |
|---|---|
| Boiling Point | 421.3ºC at 760mmHg |
| Molecular Formula | C16H19N3S |
| Molecular Weight | 285.40700 |
| Flash Point | 208.6ºC |
| Exact Mass | 285.13000 |
| PSA | 44.67000 |
| LogP | 3.69940 |
| Vapour Pressure | 2.63E-07mmHg at 25°C |
| Index of Refraction | 1.62 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
482-15-5 |
| Literature: Yale; Sowinski Journal of the American Chemical Society, 1958 , vol. 80, p. 1651,1653 |
|
~% 
482-15-5 |
| Literature: Yale; Sowinski Journal of the American Chemical Society, 1958 , vol. 80, p. 1651,1653 |
|
~% 
482-15-5 |
| Literature: Yale; Sowinski Journal of the American Chemical Society, 1958 , vol. 80, p. 1651,1653 |
|
~% 
482-15-5 |
| Literature: Yale; Sowinski Journal of the American Chemical Society, 1958 , vol. 80, p. 1651,1653 |
|
~% 
482-15-5 |
| Literature: Yale; Sowinski Journal of the American Chemical Society, 1958 , vol. 80, p. 1651,1653 |
|
~% 
482-15-5 |
| Literature: Schuler,W.A.; Klebe,H. Justus Liebigs Annalen der Chemie, 1962 , vol. 653, p. 172 - 180 |
|
~% 
482-15-5 |
| Literature: Schuler,W.A.; Klebe,H. Justus Liebigs Annalen der Chemie, 1962 , vol. 653, p. 172 - 180 |
| Precursor 7 | |
|---|---|
| DownStream 0 | |