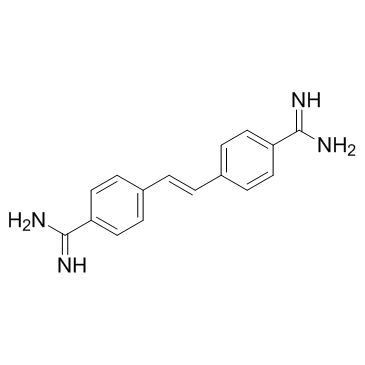
122-06-5
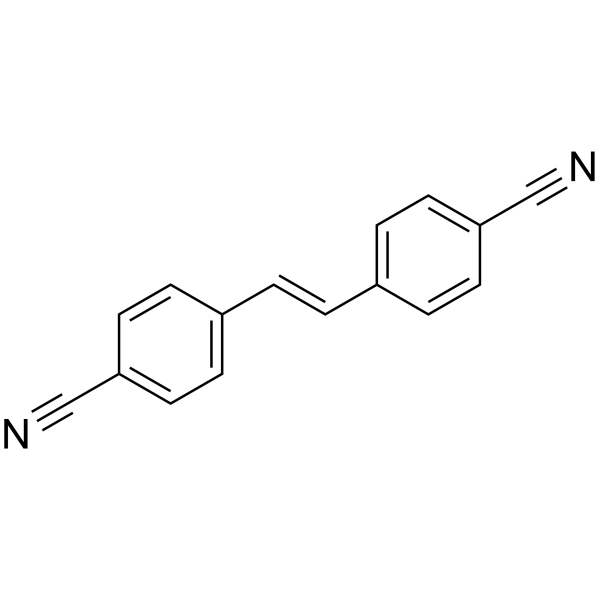
| Name | 2-(2-phenylethenyl)benzenecarboximidamide |
|---|---|
| Synonyms |
Stilbamidin
Benzenecarboximidamide,4,4'-(1,2-ethenediyl)bis 4,4'-Vinylenedi(benzamidine) stilbamidine trans-stilbene-4,4'-dicarboxamidine trans-Stilben-4,4'-dicarbamidin trans-Stilben-dicarbamidin-(4.4') 4,4'-Stilbenedicarboxamidine(7CI,8CI) |
| Description | Stilbamidine is a diamidine compound derived from Stilbene and used chiefly in the form of its crystalline isethionate salt in treating various fungal infections. |
|---|---|
| Related Catalog | |
| Target |
fungal |
| In Vitro | The high-affinity pentamidine transporter (HAPT1) is inhibited by Propamidine but displays only low affinity to Stilbamidine. adenosine-sensitive pentamidine transporter (ASPT1), in contrast, is strongly inhibited by Stilbamidine, and Propamidine. [3H]pentamidine uptake is determined in the presence of various concentrations of adenosine (IC50=1.2 μM) or melarsen oxide (IC50=0.7 μM), as well as in the presence of 250 μM adenosine and increasing concentrations of hypoxanthine, Propamidine (IC50=6.1 μM) and Stilbamidine (IC50=110 μM)[1]. The two diamidine compounds, Stilbamidine and Pentamidine are used to treat in multiple myeloma, a disease in which increase of the globulin content of the serum is of frequent occurrence[2]. |
| References |
| Density | 1.19g/cm3 |
|---|---|
| Boiling Point | 456.4ºC at 760mmHg |
| Molecular Formula | C16H16N4 |
| Molecular Weight | 264.32500 |
| Flash Point | 229.8ºC |
| Exact Mass | 264.13700 |
| PSA | 99.74000 |
| LogP | 4.02520 |
| Vapour Pressure | 1.62E-08mmHg at 25°C |
| Index of Refraction | 1.634 |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Precursor 8 | |
|---|---|
| DownStream 1 | |