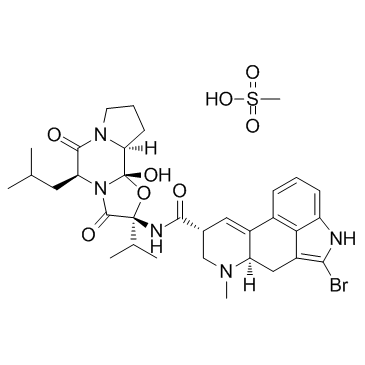
22260-51-1
| Name | bromocriptine methanesulfonate |
|---|---|
| Synonyms |
(5'α)-2-bromo-12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)-3',6',18-trioxoergotaman methanesulfonate (salt)
Bromocriptine mesilate (6aR,9R)-5-bromo-N-[(2R,5S,10aS,10bS)-10b-hydroxy-2-(1-methylethyl)-5-(2-methylpropyl)-3,6-dioxooctahydro-8H-[1,3]oxazolo[3,2-a]pyrrolo[2,1-c]pyrazin-2-yl]-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinoline-9-carboxamide methanesulfonate (salt) CB-154 mesylate acide méthanesulfonique - (6aR,9R)-5-bromo-N-[(2R,5S,10aS,10bS)-10b-hydroxy-2-(1-méthyléthyl)-5-(2-méthylpropyl)-3,6-dioxooctahydro-8H-[1,3]oxazolo[3,2-a]pyrrolo[2,1-c]pyrazin-2-yl]-7-méthyl-4,6,6a,7, Apo-Bromocriptine 2-Bromine-a-ergocryptine Methanesulfonate Parlodel 2-Bromo α-Ergocryptine Mesylate (5'α)-2-bromo-12'-hydroxy-5'-(2-methylpropyl)-3',6',18-trioxo-2'-(propan-2-yl)ergotaman methanesulfonate (1:1) hydroindolo[4,3-fg]chinolin-9-carboxamid(1:1) acide méthanesulfonique - (6aR,9R)-5-bromo-N-[(2R,5S,10aS,10bS)-10b-hydroxy-2-(1-méthyléthyl)-5-(2-méthylpropyl)-3,6-dioxooctahydro-8H-[1,3]oxazolo[3,2-a]pyrrolo[2,1-c]pyrazin-2-yl]-7-méthyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinoléine-9-carboxamide (1:1) Methansulfonsäure--(6aR,9R)-5-brom-N-[(2R,5S,10aS,10bS)-10b-hydroxy-2-(1-methylethyl)-5-(2-methylpropyl)-3,6-dioxooctahydro-8H-[1,3]oxazolo[3,2-a]pyrrolo[2,1-c]pyrazin-2-yl]-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]chinolin-9-carboxamid(1:1) EINECS 244-881-1 2-Bromo-12'-hydroxy-5'a-isobutyl-2'-isopropylergotaman-3',6',18-trione Monomethanesulphonate (5'α)-2-Bromo-12'-hydroxy-5'-isobutyl-2'-isopropyl-3',6',18-trioxoergotaman methanesulfonate (1:1) Ergotaman, 2-bromo-12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)-3',6',18-trioxo-, (5'α)-, methanesulfonate (1:1) (salt) (6aR,9R)-5-bromo-N-[(2R,5S,10aS,10bS)-10b-hydroxy-2-(1-methylethyl)-5-(2-methylpropyl)-3,6-dioxooctahydro-8H-[1,3]oxazolo[3,2-a]pyrrolo[2,1-c]pyrazin-2-yl]-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg Methansulfonsäure--(6aR,9R)-5-brom-N-[(2R,5S,10aS,10bS)-10b-hydroxy-2-(1-methylethyl)-5-(2-methylpropyl)-3,6-dioxooctahydro-8H-[1,3]oxazolo[3,2-a]pyrrolo[2,1-c]pyrazin-2-yl]-7-methyl-4,6,6a,7,8,9-hexa MFCD00069218 8,9-hexahydroindolo[4,3-fg]quinoléine-9-carboxamide (1:1) Bromocriptine (mesylate) |
| Description | Bromocriptine mesylate is a potent dopamine D2/D3 receptor agonist, which binds D2 dopamine receptor with pKi of 8.05±0.2. |
|---|---|
| Related Catalog | |
| Target |
pKi: 8.05±0.2 (dopamine D2 receptor)[1] |
| In Vitro | Bromocriptine stimulates [35S]-GTPγS binding at D2 dopamine receptor expressed in CHO cells with pEC50 of 8.15±0.05[1]. Bromocriptine also is a strong inhibitor of brain nitric oxide synthase. The ergot alkaloid Bromocriptine (BKT) is found to act as a strong inhibitor of purified neuronal nitric oxide synthase (NOS) (IC50=10±2 μM) whereas it is poorly active towards inducible macrophage NOS (IC50>100 μM) [2]. Bromocriptine is found to inhibit the activity of at least one human cytochrome P450 enzyme. Bromocriptine is a potent inhibitor of CYP3A4 with a calculated IC50 value for the interaction of 1.69 μM[3]. |
| In Vivo | Bromocriptine mesylate (2 mg/kg, i.p.) is administered for 7 days in groups of mice in forced swimming test (FST) and tail suspension test (TST). Bromocriptine group shows significant anti-immobility action as compared to control. When Bromocriptine administered 30 min after the last dose of 7 days MPE treatment and subjected to FST, this dopaminergic agonist produces significant and dose dependent potentiation of anti-immobility action of MPE (200 mg/kg, p.o.) as compared to MPE treatment alone. Bromocriptine treatment group shows a significant reduction of immobility time as compared to control. Bromocriptine administration after 7 days pretreatment with MPE (100 and 200 mg/kg, p.o.) shows significant and dose dependent potentiation of anti-immobility action of MPE as compared to MPE treatment alone[4]. Intracisternal administration of Bromocriptine decreases significantly the static mechanical allodynia (SMA) score compared to that of sham (saline-injected rats) and its effect lasted for 30 min. Intraperitoneal administration of Bromocriptine induces a significant, dose dependent (0.1 mg and 1 mg/kg) decrease in pain scores in CCI-IoN group when compared to sham and its effect lasted for 6 h. The highest dose induces the highest score decrease (P<0.01). Bromocriptine effect lasts for 20 min. Intraperitoneal administration of Bromocriptine induces a significant dose dependent decrease in SMA score in CCI-IoN+6-OHDA lesioned group compared to that of sham. Its effect lasts for 6 h[5]. |
| Kinase Assay | The [35S]-GTPγS binding assay is carried out. Cell membranes (25 ±75 ug) are incubated in Buffer B containing 0.1 mM dithiothreitol (DTT) and 1 uM GDP and drugs in a volume of 0.9 mL for 30 min at 30°C. This preincubation ensures that the agonists tested are at equilibrium when the [35S]-GTPγS (50±150 pM, final concentration) is added (in 100 uL ofBuffer B) to initiate the reaction. The assay mixture is incubated for a further 20 min unless otherwise stated. The assays are terminated by rapid filtration and bound radio-activity determined as described for the radio-ligand binding assays above. The total binding of [35S]-GTPγS is less than 20% of that added[1]. |
| Animal Admin | Mice[4] Swiss mice (20-25 g) of either sex (total 150) are used. Bromocriptine mesylate is used as dopamine receptor (D2) agonist. Haloperidol is diluted in distilled water which is used for a vehicle of injection. Bromocriptine mesylate is dissolved in one drop of glacial acetic acid and made up to volume in distilled water. Imipramine is dissolved in 0.9% normal saline. Haloperidol (0.1 mg/kg, i.p.) and Bromocriptine mesylate (2 mg/kg, i.p.) are administered for 7 days in groups of mice in Forced Swimming Test (FST) and Tail Suspension Test (TST). Imipramine (10 mg/kg, p.o.) as a standard is administered in positive control groups for 7 days. Rats[5] Adult male Sprague-Dawley rats (N=112, 275-325 g) are used. Two weeks after the 6-OHDA injection, the animals are briefly (<3 min) anesthetized with 2 % halothane using a mask and received for intracisternal administration Bromocriptine (7 μg/kg dissolved in 5 μL vehicle) or the vehicle alone (5 μL of 0.9 % saline). For i.p. injection we used Bromocriptine (1 mg/kg) and SKF81297 (3 mg/kg dissolved in 0.9 % saline) concentrations. Following a recovery period (<2 min), the rats are placed in the observation field for 40 min period-test by a blind-experimenter. |
| References |
| Boiling Point | 891.3ºC at 760 mmHg |
|---|---|
| Molecular Formula | C33H44BrN5O8S |
| Molecular Weight | 750.700 |
| Flash Point | 492.8ºC |
| Exact Mass | 749.209412 |
| PSA | 180.96000 |
| LogP | 3.98220 |
| Vapour Pressure | 0mmHg at 25°C |
| Water Solubility | H2O: 0.8 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H410 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | UN 3077 9 / PGIII |
| WGK Germany | 3 |
| RTECS | KE1595000 |