58493-49-5
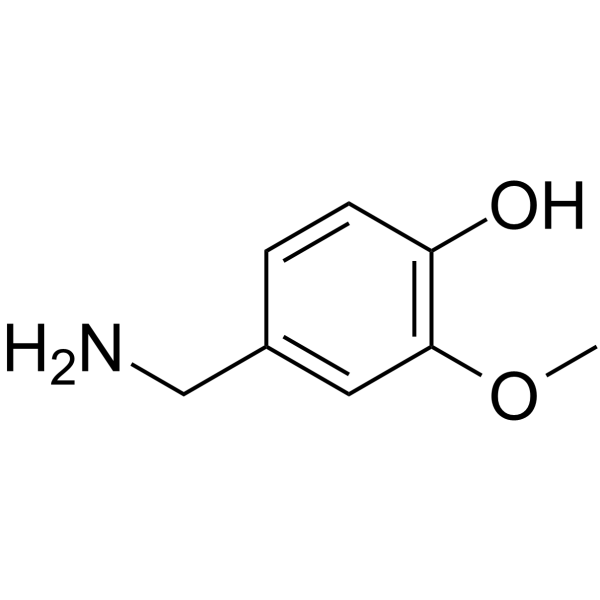
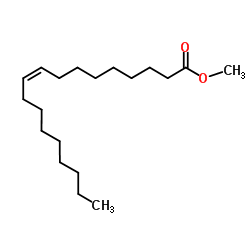
| Name | (Z)-N-[(4-hydroxy-3-methoxyphenyl)methyl]octadec-9-enamide |
|---|---|
| Synonyms |
N-vanillylamide
oleoyl vanillylamide TCMDC-124289 N-Vanillyloleamide Vanillyloleamide MFCD00673962 (9Z)-N-(4-Hydroxy-3-methoxybenzyl)-9-octadecenamide N-Vannilyloleoylamide OLVANIL (9Z)-N-(4-Hydroxy-3-methoxybenzyl)octadec-9-enamide |
| Description | Olvanil (NE-19550)is an agonist of transient receptor potential vanilloid type 1 (TRPV1) channels with an EC50 of 0.7 nM.Analgesic[1]. |
|---|---|
| Related Catalog | |
| Target |
TRPV1:0.7 nM (EC50) |
| In Vitro | Olvanil affects C6 glioma cell proliferation (IC50 value of 5.5 μM)[2] |
| In Vivo | Olvanil is one capsaicin analog, which acts as an agonist at the vanilloid receptor. Olvanil may have causes an anxiogenic-like effect. Doses of 0, 0.2, 1.0 and 5.0 mg/kg Olvanil, respectively, yielded percent open arm entries at 5 min of 25±10.1, 19.3±7.1, 14.9±5.9 and 0±0[3]. Animal Model: Sprague-Dawley rats weighing approximately 200 g[3] Dosage: 0, 0.2, 1.0 and 5.0 mg/kg Administration: Injected intraperitoneally 30 min before the behavioral tests Result: The percent open arm times at 5 min were 12.9±8.1 for the 0 mg/kg dose, 8.9±4.2 for the 0.2 mg/kg dose, 15.2±7.9 for the 1 mg/kg dose and 0±0 for the 5 mg/kg dose. The mean number of entries into the closed arm at 5 min were 1.7±0.3, 3.3±0.8, 2.7±0.3 and 0.25±0.1 for doses of 0, 0.2, 1 and 5 mg/kg, respectively. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 596.1±50.0 °C at 760 mmHg |
| Molecular Formula | C26H43NO3 |
| Molecular Weight | 417.62 |
| Flash Point | 314.3±30.1 °C |
| Exact Mass | 417.324280 |
| PSA | 58.56000 |
| LogP | 7.69 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.509 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | RG2242130 |