407-41-0
| Name | O-phospho-L-serine |
|---|---|
| Synonyms |
dexfosfoserine
O-Phospho-L-serine 3-O-(phospho)-L-serine P-serine L-O-phosphoserinate DL-SERINE PHOSPHATE HYDRATE O-Phosphoserine O-phosphoryl-L-serine L-(+)-phosphoserine O-Phosphono-L-serine Seriphos P-ser L-serine-O-phosphate 3-P-SERINE L-O-(RG) L-Serine Phosphate L-O-serine phosphate L-O-Phosphoserine EINECS 206-986-0 L-SOP H-SER(P)-OH Fosforina MFCD00065935 Phosphoserine |
| Description | O-Phospho-L-serine is the immediate precursor to L-serine in the serine synthesis pathway, and an agonist at the group III mGluR receptors (mGluR4, mGluR6, mGluR7, and mGluR8); O-Phospho-L-serine also acts as a weak antagonist for mGluR1 and a potent antagonist for mGluR2. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | O-Phospho-L-serine (l-SOP) weakly binds to mGluR1, and antagonizes the effects of l-glutamate. l-SOP activates the group III receptors (mGluR4, mGluR6, mGluR7, and mGluR8), but mGluR7 has much lower affinity for l-SOP than the other group III receptors and also displays lower efficacy for both ligands[1]. O-Phospho-L-serine (l-SOP) generates enhanced intracellular calcium responses in mGluR4 transfected cells. l-SOP inhibits the l-glutamate mediated mGluR1 response, with a Ki of 1 mM; l-SOP displays a substantially more potent inhibition of mGluR2 activation, with a Ki of 1 μM, three orders-of-magnitude more potent than for mGluR1. l-SOP induces membrane potential changes in HEK/TRPC4 cells transfected with mGluR4 or mGluR6. l-SOP induces TRPC4β activation mediated by Gαi/o proteins[2]. O-Phospho-L-serine (L-SOP) inhibits Müller glia proliferation, without affecting light-induced photoreceptor cell death. L-SOP disrupts Müller glia proliferation subsequent to or in parallel with the activation of ascl1a and stat3 expression in the light-damaged retina. L-SOP inhibits cone cell regeneration in the light-damaged retina[3]. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 475.4±55.0 °C at 760 mmHg |
| Melting Point | 190 °C(lit.) |
| Molecular Formula | C3H8NO6P |
| Molecular Weight | 185.072 |
| Flash Point | 241.3±31.5 °C |
| Exact Mass | 185.008926 |
| PSA | 139.89000 |
| LogP | -1.86 |
| Vapour Pressure | 0.0±2.6 mmHg at 25°C |
| Index of Refraction | 1.552 |
| Storage condition | Store at 0°C |
| Water Solubility | H2O: 50 mg/mL hot, clear, colorless to slightly yellow |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| Risk Phrases | 21/22 |
| Safety Phrases | 36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2931900090 |
|
~60% 
407-41-0 |
| Literature: US2013/29920 A1, ; Paragraph 0700 ; |
|
~87% 
407-41-0 |
| Literature: Journal of the American Chemical Society, , vol. 110, p. 2237 |
|
~% 
407-41-0 |
| Literature: Bioorganic Chemistry, , vol. 38, # 2 p. 74 - 80 |
|
~% 
407-41-0 |
| Literature: Journal of Biological Chemistry, , vol. 106, p. 595,599 |
|
~% 
407-41-0 |
| Literature: Journal of Biochemistry (Tokyo, Japan), , vol. 29, p. 292,299 |
|
~% 
407-41-0
Detail
|
| Literature: Advanced Synthesis and Catalysis, , vol. 349, # 8-9 p. 1349 - 1352 |
|
~% 
407-41-0 |
| Literature: Acta Chemica Scandinavica (1947-1973), , vol. 11, p. 1232,1235 |
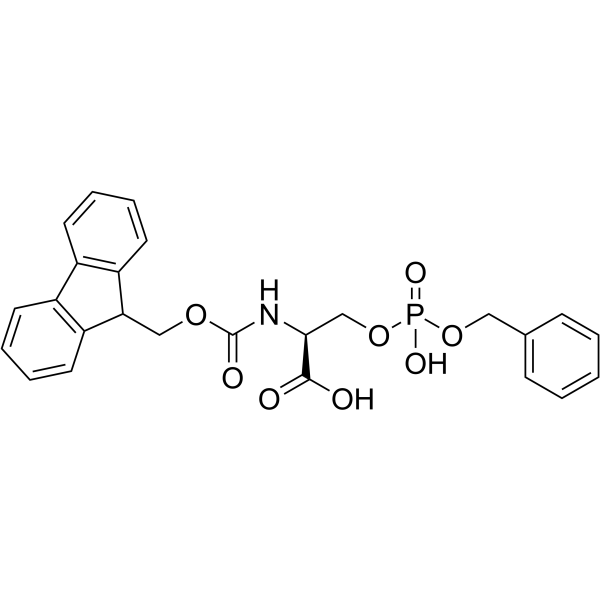
| Precursor 4 | |
|---|---|
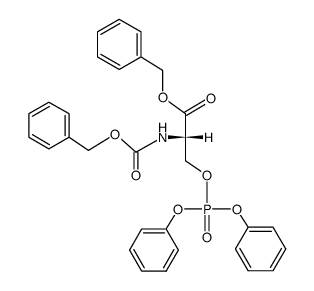
| DownStream 1 | |
| HS Code | 2931900090 |
|---|---|
| Summary | 2931900090. other organo-inorganic compounds. VAT:17.0%. Tax rebate rate:13.0%. Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward). MFN tariff:6.5%. General tariff:30.0% |