97322-87-7
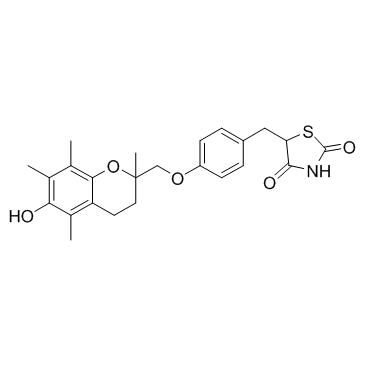
| Name | troglitazone |
|---|---|
| Synonyms |
Romglizone
5-{4-[(6-Hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-chromen-2-yl)methoxy]benzyl}-1,3-thiazolidine-2,4-dione Rezulin (TN) Rezulin 5-[4-(6-hydroxy-2,5,7,8-tetramethylchroman-2-yl-methoxy)-benzyl]thiazolidine-2,4-dione MFCD00878416 5-[(4-{[(6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-chromen-2-yl)methyl]oxy}phenyl)methyl]-1,3-thiazolidine-2,4-dione 5-[4-[(6-hydroxy-2,5,7,8-tetramethylchroman-2-yl)methoxy]-benzyl]-2,4-thiazolidinedione CS-045 3-phenyl-5,6,7,8-tetrahydroimidazo[1,2-a]pyrazine Noscal Prelay [14C]-Troglitazone 5-[4-(6-hydroxy-2,5,7,8-tetramethylchroman-2-ylmethoxy)benzyl]-2,4-dioxothiazolidine 5-[[4-[(6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydrochromen-2-yl)methoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione Romozin Troglitazone [3H]-Troglitazone Resulin |
| Description | Troglitazone is a PPARγ agonist, with EC50s of 550 nM and 780 nM for human and murine PPARγ receptor, respectively. |
|---|---|
| Related Catalog | |
| Target |
PPARγ:550 nM (EC50, Human PPARγ) |
| In Vitro | Troglitazone is a PPARγ agonist, with EC50s of 550 nM and 780 nM for human and murine PPARγ receptor, respectively[1]. Troglitazone (2-200 μM) is cytotoxic to the pancreatic cancer cell lines (MIA Paca2 and PANC-1 cells), with IC50s of 49.9 ± 1.2 and 51.3 ± 5.3 μM, respectively. Troglitazone (50 μM) increases chromatin condensation in MIA Paca2 and PANC-1 cells, enhances the activity of caspase-3 and decreases Bcl-2 expression[2]. Troglitazone (0, 1, 2, and 4 μM) sensitizes TRAIL-mediated apoptosis in human lung adenocarcinoma cells. Troglitazone enhancement of TRAIL-induced apoptosis is blocked by inhibition of autophagy, via activation of autophagy flux. In addition, the effects of troglitazone are induced by PPARγ activation in A549 cells[3]. |
| In Vivo | Troglitazone (200 mg/kg, p.o.) shows inhibitory effects on the growth of tumor in the MIA Paca2 xenograft model[2]. |
| Cell Assay | Briefly, cells are seeded into 96-well plates at a density of 1 × 105 cells/well and incubated for 24 h. The cells are treated with Troglitazone in the presence or absence of other chemicals for a further 24 h using FBS-free medium. The assay utilizes the conversion of alamar blue reagent to fluorescent resorufin by metabolically active cells. The resorufin signal is measured at an excitation wavelength of 530 nm and an emission wavelength of 580 nm. The 50% growth inhibitory concentrations (IC50) are calculated according to the sigmoid inhibitory effect model E = IC50 γ/(IC50 γ + Cγ), where E represents the surviving fraction (% of control), C represents the drug concentration in the medium, and γ represents the Hill coefficient. For co-exposure studies, the Troglitazone dosage is set to approximately the IC50 value for each cell line[2]. |
| Animal Admin | Balb/c male mice (4 weeks old) are subcutaneously inoculated in the back with MIA Paca2 cells (5 × 106 cells/100 μL in PBS) 14 days prior to starting Troglitazone administration. Mice are then orally administered 200 mg/kg Troglitazone in 0.5% methylcellulose solution or vehicle daily for 5 weeks. Tumor size is measured bi-dimensionally and the volume is calculated using the formula (length × width2) × 0.5. Body weights of mice are also monitored throughout the experiment[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 657.0±55.0 °C at 760 mmHg |
| Melting Point | 184-186°C |
| Molecular Formula | C24H27NO5S |
| Molecular Weight | 441.540 |
| Flash Point | 351.2±31.5 °C |
| Exact Mass | 441.160980 |
| PSA | 110.16000 |
| LogP | 4.99 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.608 |
| Storage condition | Store at -20°C |
| Water Solubility | DMSO: 20 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Safety Phrases | S22-S24/25 |
|---|---|
| WGK Germany | 2 |
| RTECS | XJ5813130 |
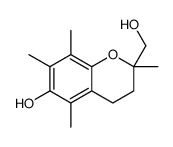
| Precursor 5 | |
|---|---|
| DownStream 0 | |

![6-Hydroxy-2,5,7,8-tetramethyl-2-[(4-nitrophenoxy)methyl]chroman structure](https://image.chemsrc.com/caspic/491/107188-58-9.png)
![6-(Methoxymethoxy)-2,5,7,8-tetramethyl-2-[(4-nitrophenoxy)methyl]chroman structure](https://image.chemsrc.com/caspic/116/118070-84-1.png)
![(+/-)-5-[4-(6-hydroxy-2,5,7,8-tetramethyl-2H-chromen-2-ylmethoxy)benzyl]thiazolidine-2,4-dione structure](https://image.chemsrc.com/caspic/413/107187-56-4.png)