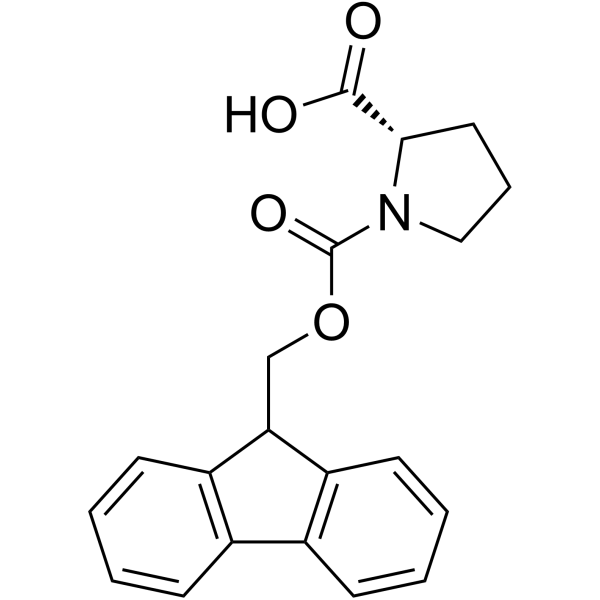
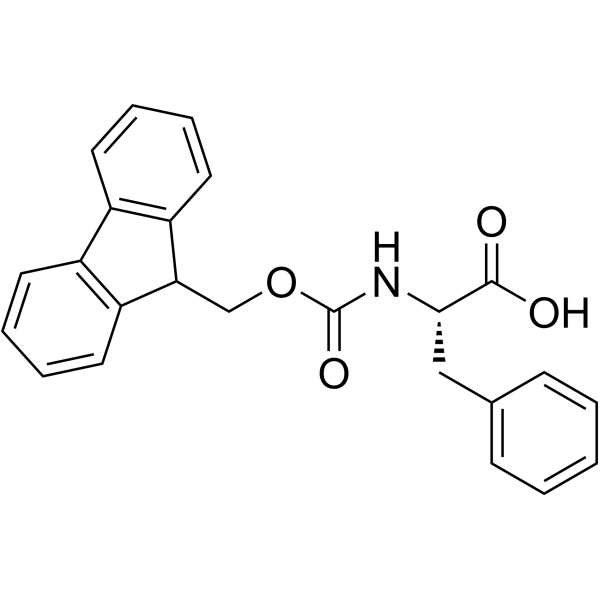
141801-26-5
| Name | Endomorphin 2 |
|---|---|
| Synonyms |
MFCD01321064
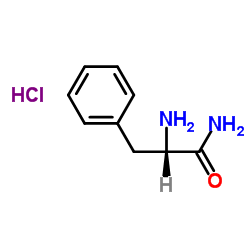
(2S)-1-[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]-N-[(2S)-1-[[(2S)-1-amino-1-oxo-3-phenylpropan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]pyrrolidine-2-carboxamide H-Tyr-Pro-Phe-Phe-NH2 TYR-PRO-PHE-PHE-NH2 Tetrapeptide-15 Endomorphin-2 |
| Description | Endomorphin 2, a high affinity, highly selective agonist of the μ-opioid receptor, displays reasonable affinities for kappa3 binding sites, with Ki value between 20 and 30 nM. |
|---|---|
| Related Catalog | |
| Target |
Ki: 20-30 nM (kappa3 opioid receptor)[1] |
| In Vitro | Endomorphin 2 is an endogenous opioid peptide and one of the two Endomorphins. It is a high affinity, highly selective agonist of the μ-opioid receptor, and along with Endomorphin 1 (EM-2). The two Endomorphins display reasonable affinities for kappa3 binding sites, with Ki values between 20 and 30 nM. Endomorphin 1 and Endomorphin 2 compete both μ1 and μ2 receptor sites quite potently. Endomorphins have little appreciable affinity for either delta or kappa1 binding sites, with Ki values greater than 500 nM[1]. |
| In Vivo | Both Endomorphin 1 and Endomorphin 2 are potent analgesics with peak effects seen at 10 and 15 min, respectively. All subsequent studies are performed at peak effect. Both compounds are fully active supraspinally and spinally, with no indication of ceiling effects. Endomorphin 1 is significantly more potent spinally than supraspinally and, at the spinal level, it is significantly more potent than Endomorphin 2. The response of both agents are readily reversed by naloxone. β-FNA, a highly selective μ antagonist, effectively reverses the actions of both Endomorphins. Both Endomorphin 1 and Endomorphin 2 display a profile similar to morphine. Neither compound have analgesic activity in CXBK mice at a dose which produced over 70% analgesia in control CD-1 mice[1]. |
| Kinase Assay | 125I-Endomorphin 1 or 125I-Endomorphin 2 binding (0.2 nM) is performed in potassium phosphate buffer (50 mM, pH 7.4; 0.5 mL) with MgCl2 (5 mM) at a tissue concentration of 10 mg wet weight/mL for brains or 0.06 mg protein/mL for MOR-1/CHO cells. Specific binding is determined in the presence and absence of either 1 μM of the corresponding unlabeled peptide. The entire mixture is then incubated at 25°C for 1 hr and filtered over no. 32 glass fiber filters which have been presoaked for 1 hr in 0.5% polyethylenimine and washed twice with ice cold Tris buffer using a Brandel cell harvester. The filters are then counted on a Packard Cobra gamma counter. The other opioid receptor binding assays are performed[1] . |
| Animal Admin | Mice[1] Groups of mice are treated i.c.v. with Endomorphin 1 (12 μg) or Endomorphin 2 (3 μg) 15 min before a 0.5-cc charcoal meal (2.5% gum tragacanth,10% activated charcoal in water). The mice are killed 30 min later and the distance the charcoal traveled is measured. |
| References |
| Density | 1.292g/cm3 |
|---|---|
| Boiling Point | 972.4ºC at 760mmHg |
| Melting Point | 130-131℃ |
| Molecular Formula | C32H37N5O5 |
| Molecular Weight | 571.66700 |
| Flash Point | 541.9ºC |
| Exact Mass | 571.27900 |
| PSA | 167.85000 |
| LogP | 3.31360 |
| Vapour Pressure | 0mmHg at 25°C |
| Index of Refraction | 1.632 |
| Storage condition | −20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
~% 
141801-26-5 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 16, # 12 p. 6415 - 6422 |
|
~46% 
141801-26-5 |
| Literature: Sakaguchi; Costa; Sakamoto; Shimohigashi Bulletin of the Chemical Society of Japan, 1992 , vol. 65, # 4 p. 1052 - 1056 |
|
~% 
141801-26-5 |
| Literature: Bulletin of the Chemical Society of Japan, , vol. 65, # 4 p. 1052 - 1056 |
|
~% 
141801-26-5 |
| Literature: Bulletin of the Chemical Society of Japan, , vol. 65, # 4 p. 1052 - 1056 |
|
~% 
141801-26-5 |
| Literature: Bulletin of the Chemical Society of Japan, , vol. 65, # 4 p. 1052 - 1056 |
|
~% 
141801-26-5 |
| Literature: Bulletin of the Chemical Society of Japan, , vol. 65, # 4 p. 1052 - 1056 |
|
~% 
141801-26-5 |
| Literature: J. Med. Chem., , vol. 47, # 3 p. 735 - 743 |
|
~% 
141801-26-5 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 15, # 11 p. 3883 - 3888 |
|
~% 
141801-26-5 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 18, # 4 p. 1350 - 1353 |
| Precursor 8 | |
|---|---|
| DownStream 0 | |