64048-12-0
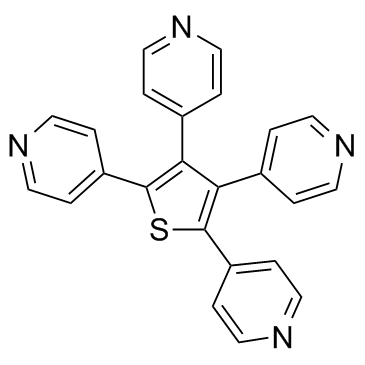
| Name | 4-(2,4,5-tripyridin-4-ylthiophen-3-yl)pyridine |
|---|---|
| Synonyms |
4,4',4'',4'''-Thiophentetrayl-tetra-pyridin
4,4',4'',4'''-thiophenetetrayl-tetra-pyridine tetra-<4-pyridyl>thiophene GANT 58 4-(2,4,5-tri(4-pyridinyl)-3-thienyl)pyridine 4,4',4'',4'''-(2,3,4,5-Thienetetrayl)tetrapyridine |
| Description | GANT 58 is a potent Gli antagonist that inhibits GLI1-induced transcription with IC50 of 5 μM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 5 μM (Gli)[1] |
| In Vitro | GANT58 is a downstream inhibitor of Hh signaling. GANT58 is an indeed inhibitor of Hh signaling downstream of Smo and Sufu. GANT58 mainly acts at the nuclear level because transcription induced by GLI1 with a mutated nuclear export signal is still blocked. GANT58 efficiently inhibits in vitro tumor cell proliferation in a GLI-dependent manner and successfully blocks cell growth using human prostate cancer cells harboring downstream activation of the Hh pathway[1]. GANT58 (NSC75503) has been shown to inhibit transcriptional activation by GLI1 (as well as by the other GLI species). GANT58 has been shown to inhibit GLI transactivation[2]. |
| In Vivo | Nude mice are injected s.c. with GLI1-positive 22Rv1 prostate cancer cells, and tumors are established (median size ≈250 mm3). Nude mice are treated with daily s.c. injections at a concentration of 50 mg/kg of cyclopamine, GANT61, GANT58, or solvent only (n=4-5 for each group). However, after 3 days, cyclopamine-treated animals presented with severe ulcerations at the injection sites. Therefore, changing the treatment regimen to injections only every second day. To be able to compare all compounds, this protocol is also introduced for the GANTs, although mice treated with these compounds showed no such signs of toxicity. All injections are done 2-3 cm away from the tumors. During an 18-day treatment period, suppression of tumor cell growth is observed for all compounds. Treatment with cyclopamine or GANT58 results in the inhibition of additional xenograft growth and limited the increase in tumor size[1]. |
| Cell Assay | HEK293 cells are transfected with GLI1 expression plasmid, together with the reporter plasmids 12× GliBS-Luc and R-Luc on 10 cm plates (day 0). Twenty-four hours later, cells are seeded in white 96 well plates with clear bottom at a density of 15,000 cells per well. Cells are allowed to attach, and compounds are added at a final concentration of 10 μM in DMSO (0.5% final DMSO concentration) (day 1.5). Cells are grown for another 24 h, subsequently lysed, and then analyzed by using the Dual Luciferase kit. Plates are read on a Berthold Technologies microplate luminometer. Subconfluent cells are grown in reduced FBS (2.5%) for 48 h in the presence of 5 μM test compound (or DMSO) on white 96 well plates with clear bottom. Subsequently, cells are labeled for 2 h with BrdU, fixed, and analyzed. Samples are read on a Molecular Devices SpectraMax Gemini EM[1]. |
| Animal Admin | Mice[1] 5×106 22Rv1 cells are suspended in a total volume of 100 μL of a 1:1 mixture of RPMI medium 1640:Matrigel (E1270). The cell suspension is injected s.c. at the posterior flank of female BALB/c nude mice (nu/nu). Tumors are grown until they reached a median size of ≈250 mm3 (5-6 days). Animals are randomly divided into four groups (n=4-5) and treated with solvent only (corn oil:ethanol, 4:1) or compounds in solvent (50 mg/kg) for 16 days s.c. injections of compounds are performed several centimeters away from the tumor. Tumor volumes are calculated by the formula length×width×0.5×(length+width). At the end of the treatment period, animals are given a BrdU pulse (50 mg/kg) for 30 min, and tumors are removed. All animal experiments are approved by local ethics authorities. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 414.1±40.0 °C at 760 mmHg |
| Molecular Formula | C24H16N4S |
| Molecular Weight | 392.476 |
| Flash Point | 169.0±17.7 °C |
| Exact Mass | 392.109558 |
| PSA | 79.80000 |
| LogP | 3.12 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.659 |
| Storage condition | 2-8℃ |
| RIDADR | NONH for all modes of transport |
|---|---|
| HS Code | 2934999090 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |