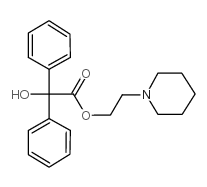
4546-39-8
| Name | 2-piperidin-1-ylethyl 2-hydroxy-2,2-diphenylacetate |
|---|---|
| Synonyms |
Pipethanatum [INN-Latin]
2-(1-Piperidino)ethyl benzilate Pipetanato [INN-Spanish] Piperilate Benzilsaeure-(2-piperidino-aethylester) pipethanate BENZILIC ACID,2-PIPERIDINOETHYL ESTER Benzilsaeure-<2-piperidino-ethylester> 1-Piperidineethanol benzilate |
| Description | Piperilate (Pipethanate) is one of the mixtures of hetrazepine derivative PAF antagonists with anticholinergics, can be used for bronchial asthma research. Piperilate also causes hypotension and rescues mice poisoned by the organophosphates[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vivo | Piperilate (11.3 mg/kg, 29.42 mg/kg; i.p.; single dose) shows anticholinergic activity, and (30 mg/kg; i.p.; single dose) exerts efficacy in mice poisoned by the organophosphates[1]. Piperilate (1 mg/kg; i.v.) causes hypotension in rabbit which is not blocked by dichloroisoproterenol or Atropine (HY-B1205)[2]. Piperilate (3 mg/kg; i.v.) decreases respiration and heart rate of test rabbits, blocks the Adrenaline (HY-122304)-induced constriction of an isolated rabbit auricle preparation[2]. Animal Model: Male albino mice (18-22 g)[1] Dosage: 11.3 mg/kg, 29.42 mg/kg Administration: Intraperitoneal injection; single dose Result: Showed anticholinergic activity with EC50 values of 11.33 mg/kg (antagonism of oxotremorine induced salivation) and 29.42 mg/kg (antagonism of oxotremorine induced tremor) in mice. |
| Density | 1.155 g/cm3 |
|---|---|
| Boiling Point | 444.1ºC at 760mmHg |
| Molecular Formula | C21H25NO3 |
| Molecular Weight | 339.42800 |
| Flash Point | 222.4ºC |
| Exact Mass | 339.18300 |
| PSA | 49.77000 |
| LogP | 2.88950 |
| Index of Refraction | 1.574 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| HS Code | 2933399090 |
|---|
|
~% 
4546-39-8 |
| Literature: Blicke; Maxwell Journal of the American Chemical Society, 1942 , vol. 64, p. 431 Full Text View citing articles Show Details Ford-Moore; Ing Journal of the Chemical Society, 1947 , p. 59 |
|
~% 
4546-39-8 |
| Literature: Blicke; Maxwell Journal of the American Chemical Society, 1942 , vol. 64, p. 431 |
|
~% 
4546-39-8 |
| Literature: Am. Cyanamid Co. Patent: US2394770 , 1942 ; |
| Precursor 5 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |