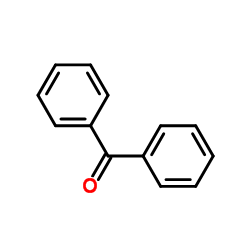
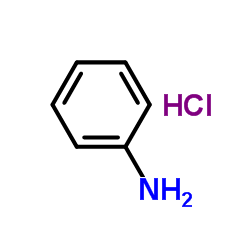
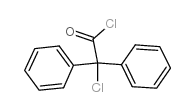
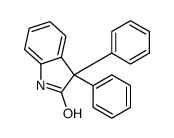
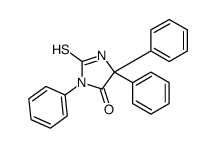
741-36-6
| Name | 1,5-bis(4-pentoxyanilino)anthracene-9,10-dione |
|---|---|
| Synonyms |
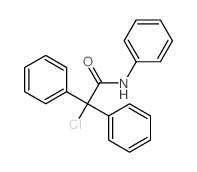
Chlorodiphenyl(9-fluorenyl)silane
9H-Fluorene,9-(chlorodiphenylsilyl) 1,1-diphenyl-1-chloroacetanilide Silane,chloro-9H-fluoren-9-yldiphenyl-(9CI) chloro-diphenyl-acetic acid anilide choro(fluoren-9-yl)diphenylsilane N-(1-chlor-1.1-diphenyl)-acetylanilin chloro-9-fluorenyldiphenylsilane 9-(CHLORODIPHENYLSILYL)-9H-FLUORENE Chloro(fluoren-9-yl)diphenylsilane Chlorodiphenyl-1-(9-fluorenyl)silane [9-(chlorodiphenylsilyl)fluorene] Chlor-diphenyl-essigsaeure-anilid |
| Density | 1.244g/cm3 |
|---|---|
| Boiling Point | 523.7ºC at 760 mmHg |
| Molecular Formula | C20H16ClNO |
| Molecular Weight | 321.80000 |
| Flash Point | 270.5ºC |
| Exact Mass | 321.09200 |
| PSA | 29.10000 |
| LogP | 4.88070 |
| Index of Refraction | 1.645 |
|
~% 
741-36-6
Detail
|
| Literature: Busby, Reginald E.; Khan, Mohammad A.; Khan, Mohammad R.; Parrick, John; Shaw, C. J. Granville Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1980 , p. 1431 - 1435 |
|
~% 
741-36-6 |
| Literature: Petjunin Zhurnal Obshchei Khimii, 1952 , vol. 22, p. 700;engl.Ausg.S.761 |
|
~% 
741-36-6 |
| Literature: Klinger Justus Liebigs Annalen der Chemie, 1912 , vol. 389, p. 250 Justus Liebigs Annalen der Chemie, 1912 , vol. 390, p. 375 |
|
~% 
741-36-6
Detail
|
| Literature: Busby, Reginald E.; Khan, Mohammad A.; Khan, Mohammad R.; Parrick, John; Shaw, C. J. Granville Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1980 , p. 1431 - 1435 |
|
~% 
741-36-6 |
| Literature: Klinger Justus Liebigs Annalen der Chemie, 1912 , vol. 389, p. 250 Justus Liebigs Annalen der Chemie, 1912 , vol. 390, p. 375 |