66085-59-4
| Name | nimodipine |
|---|---|
| Synonyms |
1-methylethyl 2-(methyloxy)ethyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
3,5-Pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 2-methoxyethyl 1-methylethyl ester Isopropyl 2-Methoxyethyl 1,4-Dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylate MFCD00153848 2,6-Dimethyl-4-(3'-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylic Acid 3-b-Methoxyethyl Ester 5-Isopropyl Ester Isopropyl 2-methoxyethyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate nimodipino 2-Methoxyethyl-1-methylethyl-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridin-3,5-dicarboxylat nimodipinum 2,6-diméthyl-4-(3-nitrophényl)-1,4-dihydropyridine-3,5-dicarboxylate de 2-méthoxyéthyle et de 1-méthyléthyle Isopropyl 2-methoxyethyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydro-3,5-pyridinedicarboxylate EINECS 266-127-0 Nimodipine 2-methoxyethyl 1-methylethyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate Isopropyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate 2-Methoxyethyl 14-Dihydro-5-(isopropoxycarbonyil)-2,6-dimethyl-4-(3-nitrophenyl)-3-pyridinecarboxylate UNII:57WA9QZ5WH |
| Description | Nimodipine(Nimotop) is a dihydropyridine derivative and an analogue of the calcium channel blocker nifedipine, with antihypertensive activity.Nimodipine decreases intracellular free Ca2+,Beclin-1 and autophagy.Target: Calcium ChannelNimodipine is main use is in the prevention of cerebral vasospasm and resultant ischemia, a complication of subarachnoid hemorrhage (a form of cerebral bleed), specifically from ruptured intracranial berry aneurysms irrespective of the patient's post-ictus neurological condition. Its administration begins within 4 days of a subarachnoid hemorrhage and is continued for three weeks. If blood pressure drops by over 5%, dosage is adjusted. There is still controversy regarding the use of intravenous nimodipine on a routine basis [1, 2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 534.8±50.0 °C at 760 mmHg |
| Melting Point | 125°C |
| Molecular Formula | C21H26N2O7 |
| Molecular Weight | 418.440 |
| Flash Point | 277.3±30.1 °C |
| Exact Mass | 418.174011 |
| PSA | 119.68000 |
| LogP | 3.86 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.539 |
| Storage condition | Store at RT |
| Water Solubility | methanol: 62.5 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H373 |
| Precautionary Statements | P260 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 1 |
| RTECS | US7975500 |
| HS Code | 2933990090 |
|
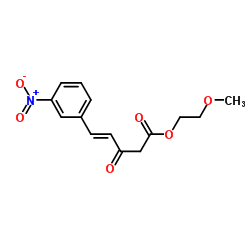
~80% 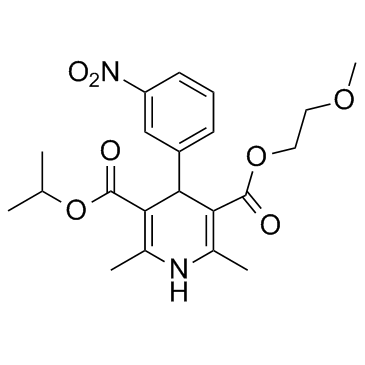
66085-59-4 |
| Literature: Meyer; Wehinger; Bossert; Scherling Arzneimittel-Forschung/Drug Research, 1983 , vol. 33, # 1 p. 106 - 112 |
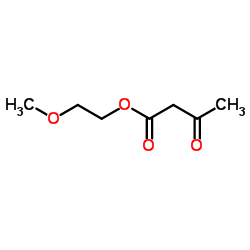
|
~% 
66085-59-4 |
| Literature: Arzneimittel-Forschung/Drug Research, , vol. 33, # 1 p. 106 - 112 |
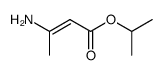
|
~70% 
66085-59-4
Detail
|
| Literature: Yiu, Sai-Hay; Knaus, Edward E. Archiv der Pharmazie, 1997 , vol. 330, # 1-2 p. 35 - 43 |
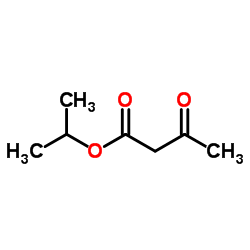
|
~% 
66085-59-4 |
| Literature: Arzneimittel-Forschung/Drug Research, , vol. 33, # 1 p. 106 - 112 |
|
~% 
66085-59-4 |
| Literature: Journal of Organic Chemistry, , vol. 61, # 3 p. 924 - 928 |
| Precursor 5 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |