136694-18-3
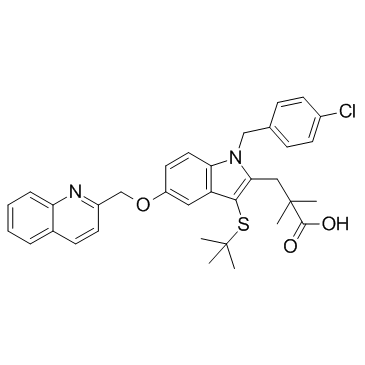
| Name | methyl 3-[3-tert-butylsulfanyl-1-[(4-chlorophenyl)methyl]-5-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethylpropanoate |
|---|---|
| Synonyms |
Methyl 3-[1-(4-chlorobenzyl)-3-[(2-methyl-2-propanyl)sulfanyl]-5-(2-quinolinylmethoxy)-1H-indol-2-yl]-2,2-dimethylpropanoate
1H-Indole-2-propanoic acid, 1-[(4-chlorophenyl)methyl]-3-[(1,1-dimethylethyl)thio]-α,α-dimethyl-5-(2-quinolinylmethoxy)-, methyl ester |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 723.8±60.0 °C at 760 mmHg |
| Melting Point | 170-172 °C |
| Molecular Formula | C35H37ClN2O3S |
| Molecular Weight | 601.198 |
| Flash Point | 391.5±32.9 °C |
| Exact Mass | 600.221313 |
| PSA | 78.65000 |
| LogP | 8.73 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.602 |
|
~% 
136694-18-3 |
| Literature: Frenette, Richard; Hutchinson, John H.; Leger, Serge; Therien, Michel; Brideau, Christine; Chan, Chi C.; Charleson, Stella; Ethier, Diane; Guay, Jocelyne; Jones, Tom R.; McAuliffe, Malia; Piechuta, Hanna; Riendeau, Denis; Tagari, Philip; Girard, Yves Bioorganic and Medicinal Chemistry Letters, 1999 , vol. 9, # 16 p. 2391 - 2396 |
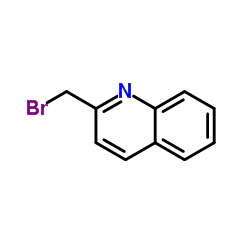
| Precursor 2 | |
|---|---|
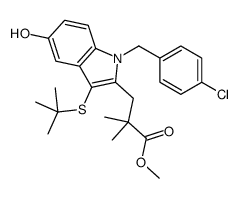
| DownStream 1 | |