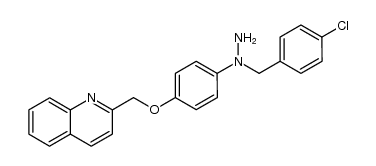
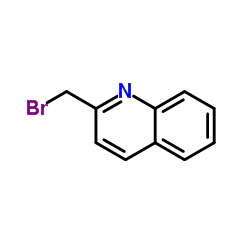
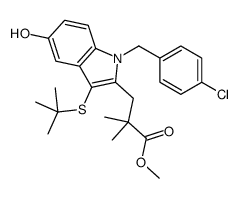
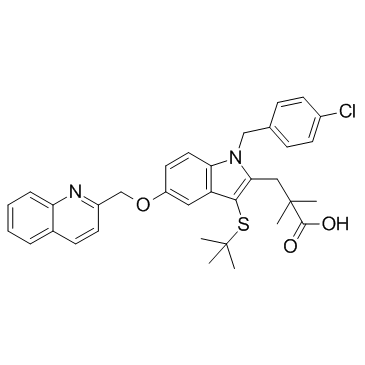
136668-42-3
| Name | 3-[3-tert-butylsulfanyl-1-[(4-chlorophenyl)methyl]-5-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethylpropanoic acid |
|---|---|
| Synonyms |
quiflapon
3-[1-[(4-chlorophenyl)methyl]-5-(quinolin-2-ylmethoxy)-3-tert-butylsulfanyl-indol-2-yl]-2,2-dimethyl-propanoic acid 3-[3-(tert-Butylsulfanyl)-1-(4-chlorobenzyl)-5-(quinolin-2-ylmethoxy)-1H-indol-2-yl]-2,2-dimethylpropanoic acid 3-[3-(1,1-dimethylethylthio)-5-(quinolin-2-ylmethoxy)-1-(4-chlorophenylmethyl)indol-2-yl]-2,2-methylpropionic acid 3-[3-(1,1-dimethylethylthio)-5-(quinolin-2-ylmethoxy)-1-(4-chlorophenylmethyl)indol-2-yl]-2,2-dimethylpropionic acid 3-[1-(4-Chlorobenzyl)-3-[(2-methyl-2-propanyl)sulfanyl]-5-(2-quinolinylmethoxy)-1H-indol-2-yl]-2,2-dimethylpropanoic acid Quiflapon [INN] 1-((4-Chlorophenyl)methyl)-3-((1,1-dimethylethyl)thio)-a,a-dimethyl-5-(2-quinolinylmethoxy)-1H-indole-2-propanoic Acid 3-[N-(p-chlorobenzyl)-3-(t-butylthio)-5-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethylpropanoic acid MK 0591 3-(tert-Butylthio)-1-(p-chlorobenzyl)-a,a-dimethyl-5-(2-quinolylmethoxy)indole-2-propionic Acid MK-0591 |
| Description | MK591 (free acid) is a selective and specific 5-Lipoxygenase-activating protein (FLAP) inhibitor with an IC50 value of 1.6 nM in a FLAP binding assay. |
|---|---|
| Related Catalog | |
| Target |
IC50 value: 1.6 nM (FLAP)[1]. |
| In Vitro | MK591 (free acid) is a potent inhibitor of leukotriene (LT) biosynthesis in intact human and elicited rat polymorphonuclear leukocytes (PMNLs) (IC50 values 3.1 and 6.1 nM, respectively) and in human, squirrel monkey, and rat whole blood (IC50 values 510, 69, and 9 nM, respectively). MK591 (free acid) has no effect on rat 5-lipoxygenase. MK591 (free acid) has a high affinity for 5-lipoxygenase activating protein (FLAP) as evidenced by an IC50 value of 1.6 nM in a FLAP binding assay and inhibition of the photoaffinity labelling of FLAP by two different photoaffinity ligands. Inhibition of activation of 5-lipoxygenase was shown through inhibition of the translocation of the enzyme from the cytosol to the membrane in human PMNLs[1]. |
| In Vivo | MK591 (free acid) is a potent inhibitor of LT biosynthesis in vivo, first, following ex vivo challenge of blood obtained from treated rats and squirrel monkeys, second, in a rat pleurisy model, and, third, as monitored by inhibition of the urinary excretion of LTE4 in antigen-challenged allergic sheep. Inhibition of antigen-induced bronchoconstriction by MK591 (free acid) is observed in inbred rats pretreated with methysergide, Ascaris-challenged squirrel monkeys, and Ascaris-challenged sheep (early and late phase response) [1]. Pups were treated with either vehicle or MK591 (free acid) 10, 20, or 40 mg/kg subcutaneously daily for days 1-4, 5-9, or 10-14. On day 14, the lungs were inflated, fixed, and stained for histopathological and morphometric analyses. Hyperoxia groups treated with MK-0591 (free acid) untreated hyperoxia groups showed definite evidence of aberrant alveolarization but no inflammation[2]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 751.3±60.0 °C at 760 mmHg |
| Molecular Formula | C34H35ClN2O3S |
| Molecular Weight | 587.171 |
| Flash Point | 408.2±32.9 °C |
| Exact Mass | 586.205688 |
| PSA | 89.65000 |
| LogP | 8.31 |
| Vapour Pressure | 0.0±2.6 mmHg at 25°C |
| Index of Refraction | 1.617 |
| Storage condition | 2-8℃ |
|
~% 
136668-42-3 |
| Literature: Merck Frosst Canada, Inc. Patent: US5254541 A1, 1993 ; Title/Abstract Full Text Show Details Merck Frosst Canada, Inc. Patent: US5254567 A1, 1993 ; |
|
~% 
136668-42-3 |
| Literature: Frenette, Richard; Hutchinson, John H.; Leger, Serge; Therien, Michel; Brideau, Christine; Chan, Chi C.; Charleson, Stella; Ethier, Diane; Guay, Jocelyne; Jones, Tom R.; McAuliffe, Malia; Piechuta, Hanna; Riendeau, Denis; Tagari, Philip; Girard, Yves Bioorganic and Medicinal Chemistry Letters, 1999 , vol. 9, # 16 p. 2391 - 2396 |
|
~% 
136668-42-3 |
| Literature: Frenette, Richard; Hutchinson, John H.; Leger, Serge; Therien, Michel; Brideau, Christine; Chan, Chi C.; Charleson, Stella; Ethier, Diane; Guay, Jocelyne; Jones, Tom R.; McAuliffe, Malia; Piechuta, Hanna; Riendeau, Denis; Tagari, Philip; Girard, Yves Bioorganic and Medicinal Chemistry Letters, 1999 , vol. 9, # 16 p. 2391 - 2396 |
|
~% 
136668-42-3 |
| Literature: Frenette, Richard; Hutchinson, John H.; Leger, Serge; Therien, Michel; Brideau, Christine; Chan, Chi C.; Charleson, Stella; Ethier, Diane; Guay, Jocelyne; Jones, Tom R.; McAuliffe, Malia; Piechuta, Hanna; Riendeau, Denis; Tagari, Philip; Girard, Yves Bioorganic and Medicinal Chemistry Letters, 1999 , vol. 9, # 16 p. 2391 - 2396 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |