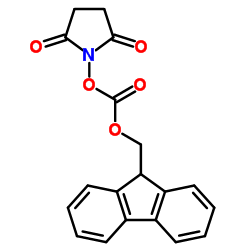
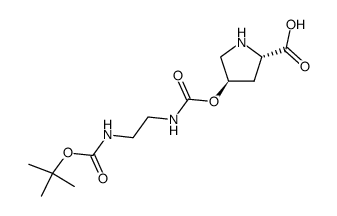
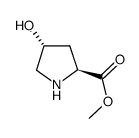
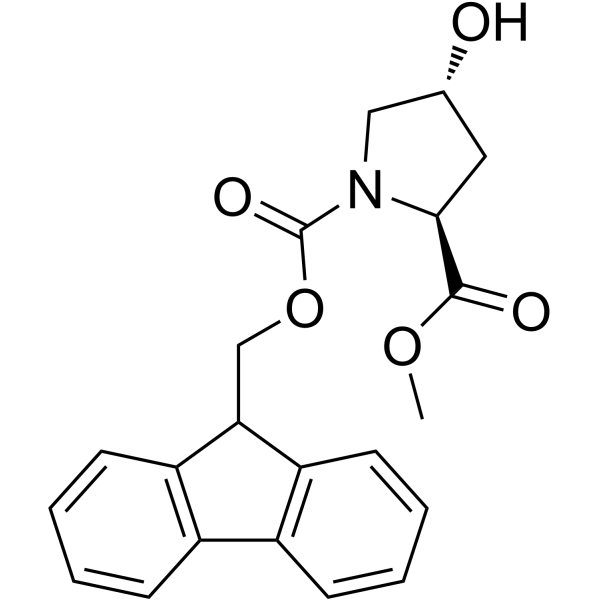
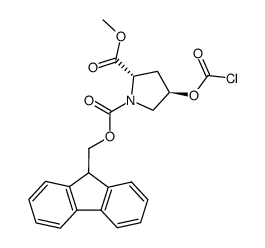
187223-15-0
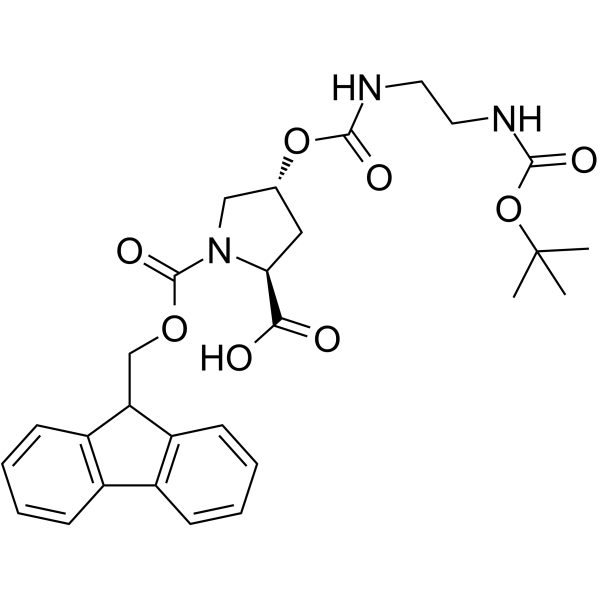
| Name | fmoc-l-hyp(bom)-oh |
|---|---|
| Synonyms |
1-[(9H-Fluoren-9-ylmethoxy)carbonyl]-4-({[2-({[(2-methyl-2-propanyl)oxy]carbonyl}amino)ethyl]carbamoyl}oxy)proline
Fmoc-(2S,4R)-Pro(4-OCO-NH-CH2-CH2-NH-Boc)-OH 1,2-Pyrrolidinedicarboxylic acid, 4-[[[[2-[[(1,1-dimethylethoxy)carbonyl]amino]ethyl]amino]carbonyl]oxy]-, 1-(9H-fluoren-9-ylmethyl) ester (4R)-1-[(9H-Fluoren-9-ylmethoxy)carbonyl]-4-({[2-({[(2-methyl-2-propanyl)oxy]carbonyl}amino)ethyl]carbamoyl}oxy)-L-proline Fmoc-Hyp(Bcc)-OH Fmoc-(2S,4R)-(4-OCO-NH-CH2-CH2-NH-Boc)-Pro-OH (2S,4R)-4-[[[[2-[[(1,1-Dimethylethoxy)carbonyl]amino]ethyl]amino]carbonyl]oxy]-1,2-pyrrolidinedicarboxylic acid 1-(9H-fluoren-9-ylmethyl) ester Fmoc-Hyp(Bom)-OH |
| Description | Fmoc-Hyp(Bom)-OH is a non-cleavable ADC linker used in the synthesis of antibody-drug conjugates (ADCs). Fmoc-Hyp(Bom)-OH is also a alkyl chain-based PROTAC linker that can be used in the synthesis of PROTACs[1]< |
|---|---|
| Related Catalog | |
| Target |
Cleavable |
| In Vitro | ADCs are comprised of an antibody to which is attached an ADC cytotoxin through an ADC linker[1]. PROTACs contain two different ligands connected by a linker; one is a ligand for an E3 ubiquitin ligase and the other is for the target protein. PROTACs exploit the intracellular ubiquitin-proteasome system to selectively degrade target proteins[2]. |
| References |
| Density | 1.35±0.1 g/cm3 |
|---|---|
| Boiling Point | 749.0±60.0 °C at 760 mmHg |
| Molecular Formula | C28H33N3O8 |
| Molecular Weight | 539.577 |
| Flash Point | 406.8±32.9 °C |
| Exact Mass | 539.226746 |
| PSA | 143.50000 |
| LogP | 3.54 |
| Vapour Pressure | 0.0±2.6 mmHg at 25°C |
| Index of Refraction | 1.620 |
| Water Solubility | Insuluble (1.5E-3 g/L) (25 ºC) |
|
~% 
187223-15-0 |
| Literature: NOVARTIS AG; NOVARTIS PHARMA GMBH Patent: WO2004/52817 A1, 2004 ; Location in patent: Page 19-20 ; |
|
~70% 
187223-15-0 |
| Literature: Lewis, Ian; Bauer, Wilfried; Albert, Rainer; Chandramouli, Nagarajan; Pless, Janos; Weckbecker, Gisbert; Bruns, Christian Journal of Medicinal Chemistry, 2003 , vol. 46, # 12 p. 2334 - 2344 |
|
~% 
187223-15-0 |
| Literature: Lewis, Ian; Bauer, Wilfried; Albert, Rainer; Chandramouli, Nagarajan; Pless, Janos; Weckbecker, Gisbert; Bruns, Christian Journal of Medicinal Chemistry, 2003 , vol. 46, # 12 p. 2334 - 2344 |
|
~% 
187223-15-0 |
| Literature: Lewis, Ian; Bauer, Wilfried; Albert, Rainer; Chandramouli, Nagarajan; Pless, Janos; Weckbecker, Gisbert; Bruns, Christian Journal of Medicinal Chemistry, 2003 , vol. 46, # 12 p. 2334 - 2344 |
|
~% 
187223-15-0 |
| Literature: Lewis, Ian; Bauer, Wilfried; Albert, Rainer; Chandramouli, Nagarajan; Pless, Janos; Weckbecker, Gisbert; Bruns, Christian Journal of Medicinal Chemistry, 2003 , vol. 46, # 12 p. 2334 - 2344 |
|
~% 
187223-15-0 |
| Literature: Lewis, Ian; Bauer, Wilfried; Albert, Rainer; Chandramouli, Nagarajan; Pless, Janos; Weckbecker, Gisbert; Bruns, Christian Journal of Medicinal Chemistry, 2003 , vol. 46, # 12 p. 2334 - 2344 |
|
~% 
187223-15-0 |
| Literature: Lewis, Ian; Bauer, Wilfried; Albert, Rainer; Chandramouli, Nagarajan; Pless, Janos; Weckbecker, Gisbert; Bruns, Christian Journal of Medicinal Chemistry, 2003 , vol. 46, # 12 p. 2334 - 2344 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |