312636-16-1
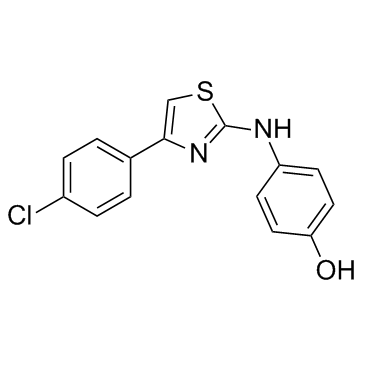
| Name | 4-[[4-(4-chlorophenyl)-1,3-thiazol-2-yl]amino]phenol |
|---|---|
| Synonyms |
4-{[4-(4-Chlorophenyl)-1,3-thiazol-2-yl]amino}phenol
4-[[4-(4-Chlorophenyl)-2-thiazolyl]amino]phenol 4-(4-(4-chloro-phenyl)thiazol-2-ylamino)phenol Kinome_2076 Phenol (4-[[4-(4-chlorophenyl)-2-thiazolyl]amino] SKI II SphK-I2,Sphingosine Kinase Inhibitor 2 UUL |
| Description | SKI-II is a synthetic inhibitor of sphingosine kinase (SK) activity with IC50 of 78 μM for SK1 and 45 μM for SK2.IC50 value: 78/45 μM (SK1/2) [2]Target: SKin vitro: SKI II inhibits cell proliferation by suppressing the Wnt/β-catenin signaling pathway. SKI II also reduces the expression of c-Myc and cyclin D1, the downstream target genes of the Wnt signaling pathway. SKI II inhibits cell proliferation by suppressing the Wnt/β-catenin signaling pathway. SKI II promotes the degradation of β-catenin by enhancing Wnt5A. SKI II inhibits the proliferation of HepG2 cells by blocking the Wnt/β-catenin signaling pathway. [1]in vivo: SKI-II causes an irreversible inhibition of SK1 by inducing its lysosomal and/or proteasomal degradation. In the present study, SKI-II was administered 3-weekly i.p. to LDL-R-/- mice for 16 weeks at a dose previously demonstrated to reduce tumor growth in mice. Preliminary experiments revealed that a single administration of SKI-II produces a significant reduction of plasma S1P with the maximum (40%) observed 12 h after injection. [2] |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 507.1±60.0 °C at 760 mmHg |
| Molecular Formula | C15H11ClN2OS |
| Molecular Weight | 302.779 |
| Flash Point | 260.5±32.9 °C |
| Exact Mass | 302.028076 |
| PSA | 73.39000 |
| LogP | 3.91 |
| Appearance | white solid |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.709 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |