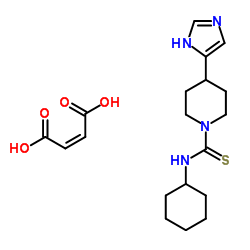
148440-81-7
| Name | Thioperamide maleate salt |
|---|---|
| Synonyms |
MR-12842 N-Cyclohexyl-4-(1H-imidazol-4-yl)-1-piperidinecarbothioamide maleate salt
1-Piperidinecarbothioamide, N-cyclohexyl-4-(1H-imidazol-5-yl)-, (2Z)-2-butenedioate (1:1) MR-12842 (N-Cyclohexyl-4-(1H-imidazol-4-yl)-1-piperidinecarbothioamide maleate salt Thioperamide Maleate N-Cyclohexyl-4-(1H-imidazol-5-yl)-1-piperidinecarbothioamide (2Z)-2-butenedioate (1:1) 1-Piperidinecarbothioamide, N-cyclohexyl-4-(1H-imidazol-4-yl)-, (2Z)-2-butenedioate (1:1) MFCD00083209 N-Cyclohexyl-4-(1H-imidazol-4-yl)piperidine-1-carbothioamide (2Z)-but-2-enedioate (1:1) |
| Description | Thioperamide maleate (MR-12842 maleate) is a potent, orally available, brain penetrant and selective H3 receptor antagonist with a Ki of 4.3 nM for inhibition of [3H]histamine release. Thioperamide maleate inhibits [3H]histamine synthesis with a Ki of 31 nM[1]. |
|---|---|
| Related Catalog | |
| Target |
H3 Receptor |
| In Vitro | Thioperamide inhibits [3H]-(R)α-MeHA binding rat brain and guinea-pig lung with Kis of 2.1 nM and 2.0 nM, respectively. Thioperamide competitively blocks H3-autoreceptors regulating [3H]histamine release with a mean apparent Ki of 4 nM[1]. Thioperamide (0.01-100 μM; 24 hours) promotes the viability of NE-4C stem cells in a concentration-dependent manner[2]. Thioperamide displays similar potencies at human H4 and H3 receptors (Ki=43 and 60 nM, respectively)[3]. Cell Viability Assay[2] Cell Line: NE-4C stem cells Concentration: 0.01, 0.1, 1, 10, 100 μM Incubation Time: 24 hours Result: The viability of NE-4C stem cells increased significantly to 150.83±6.91% when (1 μM) was administrated, and increased to 145.11±14.52% and 132.02%±25.65% when 10 μM and 100 μM were administrated respectively. |
| In Vivo | Thioperamide (5-20 mg/kg; i.p.) is able to facilitate reconsolidation of a contextually-conditioned fear memory in C57BL/6J mice[4]. Animal Model: Naive female C57BL/6J mice[4] Dosage: 5, 10 or 20 mg/kg Administration: Injections (i.p.) Result: Facilitated reconsolidation of a contextually-conditioned fear memory. |
| References |
| Molecular Formula | C19H28N4O4S |
|---|---|
| Molecular Weight | 408.515 |
| Exact Mass | 408.183136 |
| PSA | 150.64000 |
| LogP | 2.83690 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26 |
| RIDADR | NONH for all modes of transport |