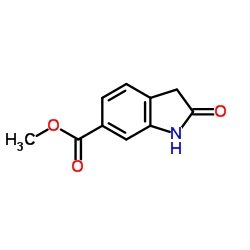
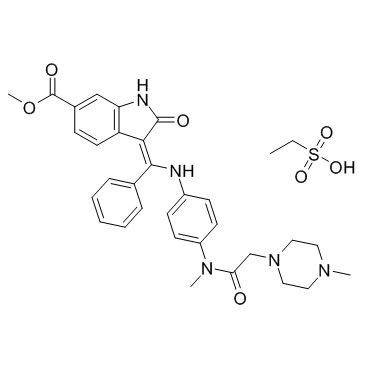
656247-17-5
| Name | nintedanib |
|---|---|
| Synonyms |
BIBF 1120
Imidafenacin [14C]-Intedanib Intedanib Vargatef Nintedanib Staybla BIBF-1120 Methyl (3Z)-3-{[(4-{methyl[(4-methyl-1-piperazinyl)acetyl]amino}phenyl)amino](phenyl)methylene}-2-oxo-6-indolinecarboxylate Ofev Uritos |
| Description | BIBF 1120 is a potent triple angiokinase inhibitor for VEGFR1/2/3, FGFR1/2/3 and PDGFRα/β with IC50s of 34 nM/13 nM/13 nM, 69 nM/37 nM/108 nM and 59 nM/65 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
VEGFR1:34 nM (IC50) VEGFR2:13 nM (IC50) VEGFR3:13 nM (IC50) FGFR1:69 nM (IC50) FGFR2:37 nM (IC50) FGFR3:108 nM (IC50) PDGFRα:59 nM (IC50) PDGFRβ:65 nM (IC50) |
| In Vitro | BIBF 1120 binds to the ATP-binding site in the cleft between the amino and carboxy terminal lobes of the kinase domain. BIBF 1120 inhibits proliferation of PDGF-BB stimulated BRPs with EC50 of 79 nM in cell assays. BIBF1120 (100 nM) blocks activation of MAPK after stimulation with 5% serum plus PDGF-BB. BIBF1120 prevents PDGF-BB stimulated proliferation with an EC50 of 69 nM in cultures of human vascular smooth muscle cells (HUASMC)[1]. |
| In Vivo | BIBF1120 (25-100 mg/kg daily p.o.) is highly active in all tumor models, including human tumor xenografts growing in nude mice and a syngeneic rat tumor model. This is evident in the magnetic resonance imaging of tumor perfusion after 3 days, reducing vessel density and vessel integrity after 5 days, and profound growth inhibition[1]. BIBF1120 is orally available and displays encouraging efficacy in in vivo tumor models while being well tolerated[2]. |
| Kinase Assay | Enzyme activity is assayed in the presence or absence of serial dilutions of BIBF1120 performed in 25% DMSO. Each microtiter plate contains internal controls such as blank, maximum reaction, and historical reference compound. All incubations are conducted at room temperature on a rotation shaker. 10 μL of each BIBF1120 dilution is added to 10 μL of diluted kinase (0.8 μg/mL VEGFR2, 10 mM Tris pH 7.5, 2 mM EDTA, and 2 mg/mL BSA) and preincubated for 1 hour. The reaction is started by addition of 30 μL of substrate mix containing 62.4 mM Tris pH 7.5, 2.7 mM DTT, 5.3 mM MnCl2, 13.3 mM Mg-acetate, 0.42 mM ATP, 0.83 mg/mL Poly-Glu-Tyr(4:1), and 1.7 μg/mL Poly-Glu-Tyr(4:1)-biotin and incubated for 1 hour. The reaction is stopped by addition of 50 μL of 250 mM EDTA, 20 mM HEPES, pH 7.4. 90 μL of the reaction mix is transferred to a streptavidin plate and incubated for 1-2 hours. After three washes with PBS the EU-labeled antibody, PY20 is added (recommended dilution 1:2000 of 0.5 mg/mL labeled antibody in DELFIA assay buffer). Excessive detection antibody is removed by three ishes of DELFIA washing buffer. Then 10 minutes before measurement on the multilabel reader, each well is incubated with 100 μL of DELFIA enhancement solution. |
| Animal Admin | Five-week-old to 6-wk-old athymic NMRI-nu/nu female mice (21-31 g) are used for the assay. After acclimatization, mice are inoculated with 1 to 5×106 (in 100 μL) FaDu, Caki-1, SKOV-3, H460, HT-29, or PAC-120 cells s.c. into the right flank of the animal. After acclimatization, F344 Fischer rats are injected with 5×106 (in 100 μL) GS-9L cells s.c. into the right flank of the animal. For pharmacokinetic analysis, blood is isolated at indicated time points from the retroorbital plexus of mice and plasma is analyzed using high performance liquid chromatography-mass spectrometry methodology. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 742.2±60.0 °C at 760 mmHg |
| Molecular Formula | C31H33N5O4 |
| Molecular Weight | 539.625 |
| Flash Point | 402.7±32.9 °C |
| Exact Mass | 539.253235 |
| PSA | 94.22000 |
| LogP | 2.59 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.658 |
|
~75% 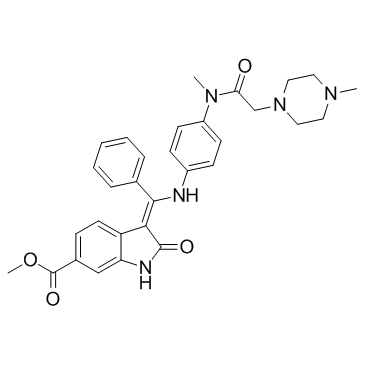
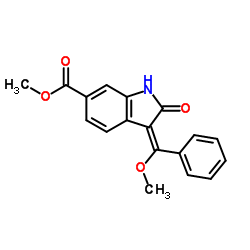
656247-17-5 |
| Literature: WO2004/13099 A1, ; Page 13 ; |
|
~77% 
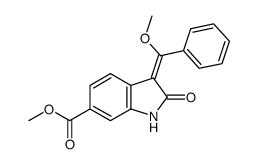
656247-17-5 |
| Literature: EP1473043 A1, ; Page 6 ; EP 1473043 A1 |
|
~91% 
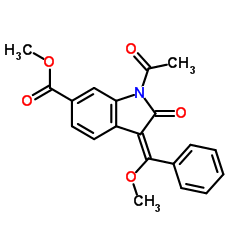
656247-17-5 |
| Literature: WO2012/68441 A2, ; Page/Page column 11 ; |
|
~87% 
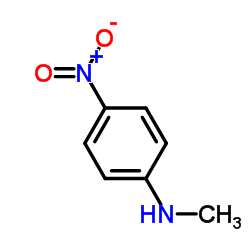
656247-17-5 |
| Literature: WO2009/71524 A2, ; Page/Page column 22-23; 13 ; |
|
~76% 
656247-17-5 |
| Literature: Journal of Medicinal Chemistry, , vol. 52, # 14 p. 4466 - 4480 |
|
~% 
656247-17-5 |
| Literature: WO2012/68441 A2, ; |
|
~% 
656247-17-5 |
| Literature: WO2012/68441 A2, ; |
|
~% 
656247-17-5 |
| Literature: WO2012/68441 A2, ; |
|
~% 
656247-17-5 |
| Literature: WO2012/68441 A2, ; |
| Precursor 6 | |
|---|---|
| DownStream 1 | |