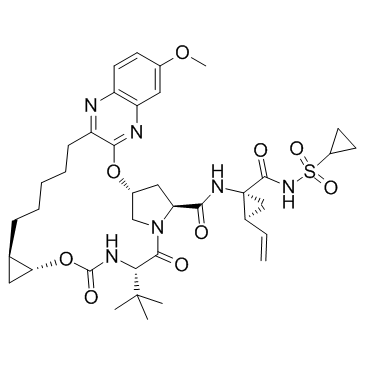
1350514-68-9
| Name | Grazoprevir |
|---|---|
| Synonyms |
(1R,18R,20R,24S,27S)-N-{(1R,2S)-1-[(Cyclopropylsulfonyl)carbamoyl]-2-vinylcyclopropyl}-7-methoxy-24-(2-methyl-2-propanyl)-22,25-dioxo-2,21-dioxa-4,11,23,26-tetraazapentacyclo[24.2.1.0.0.0]nonacosa-3,5,7,9,11-pentaene-27-carboxamide
UNII-8YE81R1X1J (1aR,5S,8S,10R,22aR)-5-tert-butyl-N-((1R,2S)-1-{[(cyclopropylsulfonyl)amino]carbonyl}-2-vinylcyclopropyl)-14-methoxy-3,6-dioxo-1,1a,3,4,5,6,9,10,18,19,20,21,22,22a-tetradecahydro-8H-7,10-methanocyclopropa[18,19][1,10,3,6]dioxadiazacyclononadecino[11,12-b]quinoxaline-8-carboxamide MK-5172 CS-1374 grazoprevir MK5172 |
| Description | Grazoprevir (MK-5172) is a selective inhibitor of Hepatitis C virus NS3/4a protease with broad activity across genotypes and resistant variants, with Kis of 0.01 nM (gt1b), 0.01 nM (gt1a), 0.08 nM (gt2a), 0.15 nM (gt2b), 0.90 nM (gt3a), respectively. |
|---|---|
| Related Catalog | |
| Target |
Ki: 0.01±<0.01 nM (gt1b), 0.01±0.01 nM (gt1a), 0.08±0.02 nM (gt2a), 0.15±0.06 nM (gt2b), 0.90±0.2 nM (gt3a)[1] |
| In Vitro | In biochemical assays, Grazoprevir (MK-5172) is effective against a panel of major genotypes and variants engineered with common resistant mutations, with Ki of 0.01±<0.01 nM (gt1b), 0.01±0.01 nM (gt1a), 0.08±0.02 nM (gt2a), 0.15±0.06 nM (gt2b), 0.90±0.2 nM (gt3a), 0.07±0.01 nM (gt1bR155K), 0.14±0.03 nM (gt1bD168V), 0.30±0.04 nM (gt1bD168Y), 5.3±0.9 nM (gt1bA156T), and 12±2 nM (gt1bA156V), respectively. In the replicon assay, Grazoprevir demonstrates subnanomolar to low-nanomolar EC50s against genotypes 1a, 1b, and 2a, with EC50s of 0.5±0.1 nM, 2±1 nM, and 2±1 nM for gt1bcon1, gt1a, and gt2a, respectively. Grazoprevir is potent against a panel of HCV replication mutants NS5A (Y93H) (EC50=0.7±0.3 nM), NS5B nucleosides (S282T) (EC50=0.3±0.1 nM), and NS5B (C316Y) (EC50=0.4±0.2)[1]. Grazoprevir (MK-5172) maintains the excellent potency against the gt 3a enzyme as well as a broad panel of mutant enzymes, has excellent potency in the replicon system [gt1b IC50(50% NHS)=7.4 nM; gt1a IC50(40% NHS)=7 nM], and shows excellent rat liver exposure[2]. |
| In Vivo | Grazoprevir (MK-5172) demonstrates efficacy in vivo against chronic-HCV-infected chimpanzees[1]. When dosed to dogs, Grazoprevir (MK-5172) shows low clearance of 5 mL/min/kg and a 3 h half-life after iv dosing and has good plasma exposure (AUC=0.4 μM h) after a 1 mg/kg oral dose. Dog liver biopsy studies showed that the liver concentration of Grazoprevir after the 1 mg/kg oral dose is 1.4 μM at the 24 h time point. Similar to its behavior in rats, Grazoprevir demonstrates effective partitioning into liver tissue and maintains high liver concentration, relative to potency, 24 h after oral dosing in dogs[2]. |
| Animal Admin | Rats and Dogs[1] Studies are performed in both rats and dogs. For studies in which Grazoprevir is dosed intravenously to rats or dogs, the compound is formulated in polyethylene glycol 200 (PEG200) and administered as a bolus at either 2 mg/kg of body weight (Rats) or 0.5 mg/kg (dog). For oral studies, the crystalline potassium salt of the compound is dosed as a solution in PEG400 at 5 mg/kg (Rats) or 1 mg/kg (dog). For all studies, blood samples are collected in EDTA-containing tubes at appropriate times and plasma is separated by centrifugation and stored at −70°C until analysis. Quantitation of Grazoprevir (MK-5172) levels is conducted by high-performance liquid chromatography/mass spectroscopy (LC/MS/MS) following protein precipitation. Liver samples are obtained from rat studies at the termination of the experiment. For dog, liver biopsy samples (20 μL) are collected following sedation. Tissue samples are homogenized in four volumes of deionized water, and drug concentrations are determined by LC/MS/MS after protein precipitation. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C38H50N6O9S |
| Molecular Weight | 766.903 |
| Exact Mass | 766.335999 |
| PSA | 203.60000 |
| LogP | 3.93 |
| Index of Refraction | 1.633 |
| Storage condition | -20℃ |
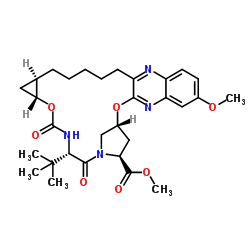
| Precursor 3 | |
|---|---|
| DownStream 0 | |