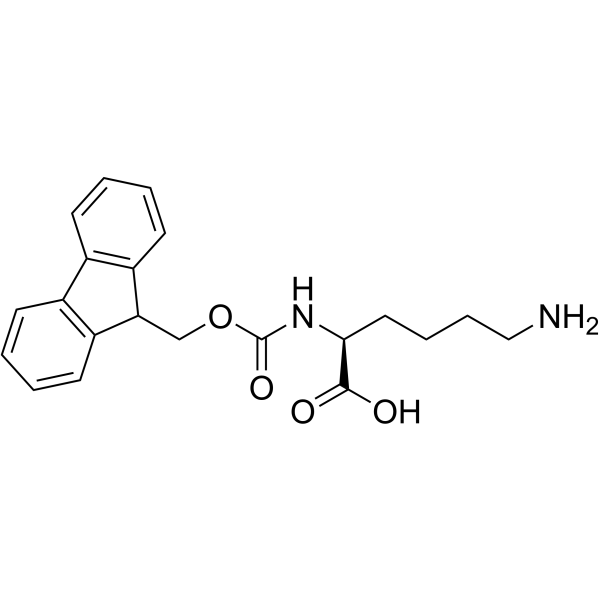
146982-27-6
| Name | Fmoc-Lys(Alloc)-OH |
|---|---|
| Synonyms |
Fmoc-Lys(Aloc)-OH
(S)-2-((((9H-fFuoren-9-yl)methoxy)carbonyl)amino)-6-(((allyloxy)carbonyl)amino)hexanoic acid (2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-6-(prop-2-enoxycarbonylamino)hexanoic acid N-[(Allyloxy)carbonyl]-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-lysine MFCD00190872 |
| Description | Fmoc-Lys(Alloc)-OH is a lysine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 689.7±55.0 °C at 760 mmHg |
| Melting Point | 87-91ºC |
| Molecular Formula | C25H28N2O6 |
| Molecular Weight | 452.500 |
| Flash Point | 370.9±31.5 °C |
| Exact Mass | 452.194733 |
| PSA | 113.96000 |
| LogP | 4.57 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.579 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2924299090 |
|
~95% 
146982-27-6 |
| Literature: Klar, Ulrich; Willuda, Joerg; Menrad, Andreas; Bosslet, Klaus Patent: US2005/234247 A1, 2005 ; Location in patent: Page/Page column 15 ; |
| Precursor 2 | |
|---|---|
| DownStream 1 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |