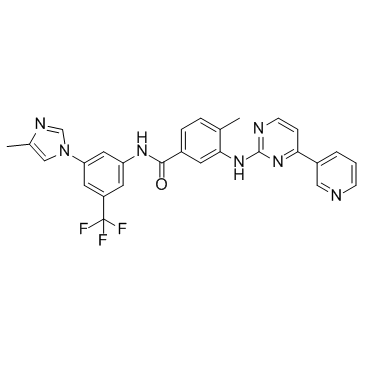
923288-90-8
| Name | 4-methyl-N-[3-(4-methylimidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]benzamide,hydrate,hydrochloride |
|---|---|
| Synonyms |
Nilotinib hydrochloride monohydrate
4-Methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-{[4-(3-pyridinyl)-2-pyrimidinyl]amino}benzamide hydrochloride hydrate Tasigna (TN) Benzamide, 4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-, hydrochloride, hydrate (1:1:1) 4-methyl-N-[3-(4-methyl-imidazol-1-yl)-5-trifluoromethyl-phenyl]-3-(4-pyridin-3-yl-pyrimidin-2-ylamino)-benzamide monohydrochloride monohydrate Nilotinib hydrochloride hydrate 5JHU0N1R6K Nilotinib (monohydrochloride monohydrate) Nilotinib hydrochloride hydrate (JAN) UNII-5JHU0N1R6K Nilotinib monohydrochloride monohydrate |
| Description | Nilotinib monohydrochloride monohydrate is a second generation tyrosine kinase inhibitor (TKI), is significantly more potent against BCR-ABL than Imatinib, and is active against many Imatinib-resistant BCR-ABL mutants. |
|---|---|
| Related Catalog | |
| Target |
Bcr-Abl[1] |
| In Vitro | The novel, selective Abl inhibitor, Nilotinib (AMN107), is designed to interact with the ATP-binding site of BCR-ABL with a higher affinity than Imatinib. In addition to being significantly more potent compared with Imatinib (IC50<30 nM), Nilotinib also maintains activity against most of the BCR-ABL point mutants that confer Imatinib resistance[1]. Nilotinib demonstrates significant antitumor efficacy against GIST xenograft lines and Imatinib-resistant GIST cell lines. The parent cell lines GK1C and GK3C show Imatinib sensitivity with IC50 of 4.59±0.97 µM and 11.15±1.48 µM, respectively. The Imatinib-resistant cell lines GK1C-IR and GK3C-IR show Imatinib resistance with IC50 values of 11.74±0.17 µM (P<0.001) and 41.37±1.07 µM (P<0.001), respectively[2]. |
| In Vivo | The percentage of tumor growth inhibition (TGI) is 83.8% for Imatinib and 69.6% for Nilotinib in the GK1X xenograft line (n.s.). In the GK2X xenograft line, TGI is 83.0% for Imatinib and 85.3% for Nilotinib (n.s.). Additionally, the GK3X xenograft line TGI is 31.1% for Imatinib and 47.5% for Nilotinib (n.s.). These results suggest that, except for the GK1X xenograft line, Nilotinib shows equivalent or higher antitumor effects than Imatinib[2]. Nilotinib has a significant healing effect on the macroscopic and microscopic pathologic scores and ensures considerable mucosal healing in the indomethacin-induced enterocolitis rat model. While Nilotinib decreased the PDGFR α and β levels and apoptotic scores in the colon, it did not have a significant effect on the weight and TNF-α levels. Further experimental investigations could provide more definitive evidence for humans[3]. |
| Cell Assay | The human GIST cell lines GK1C and GK3C, and the Imatinib-resistant cell lines GK1C-IR and GK3C-IR are plated in 96-well microplates and cultured for 12 h before exposure to Imatinib (1-100 µM) or Nilotinib (1-100 µM) for 72 h. The cells are quantified by the WST-8 assay. The optical density (OD) is determined with Sunrise rainbow. The rate of inhibition is calculated as follows: % of inhibition=(OD of treated group-blank)/(OD of control group-blank)×100%. The concentration of tested drugs resulting in 50% growth inhibition (IC50) is calculated[2]. |
| Animal Admin | Mice[2] The GIST xenograft lines GK1X, GK2X and GK3X in nude mice are used. These xenograft lines are maintained by continual passage in BALB/cSLc-nu/nu mice. Mice bearing GK1X, GK2X and GK3X tumors (6-8 mice per group) are treated daily with vehicle or 40 mg/kg Imatinib or Nilotinib for 4 weeks. Tumor volume (TV) is determined from caliper measurements of tumor length (L) and width (w) according to the formula LW2/2. TV is determined every two to three days and on the day of evaluation. Mice are sacrificed and the percentage of tumor growth inhibition (TGI) is calculated as follows: TGI (%)=[1-(mean of treatment group tumor volume on evaluation day-mean of treatment group tumor volume on day 1)/(mean of control group tumor volume on evaluation day-mean of control group tumor volume on day 1)]×100. Rats[3] Female Wistar albino rats, weighing 226-243 g (mean weight, 241.09 g), for use in this study. Nilotinib, administered 20 mg/kg/d in two divided doses, is administered to the Nilotinib group of rats (n=7) for 13 d through an orogastric tube, beginning on the same day as indomethacin administration. Blood and tissue samples for pathological examination are obtained from all rats under ether anesthesia at the end of the 13-d period. All animals are then sacrificed by decapitation. |
| References |
| Molecular Formula | C28H25ClF3N7O2 |
|---|---|
| Molecular Weight | 583.992 |
| Exact Mass | 583.171021 |
| PSA | 101.11000 |
| LogP | 6.81280 |
|
~% 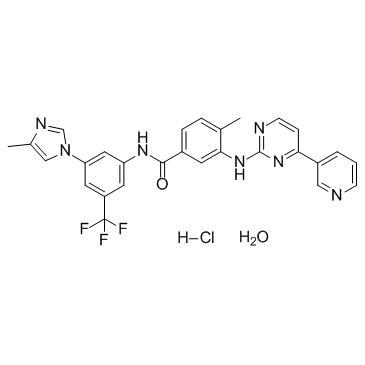
923288-90-8 |
| Literature: WO2007/15870 A2, ; Page/Page column 28 ; |
| Precursor 1 | |
|---|---|
| DownStream 0 | |