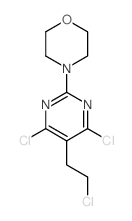
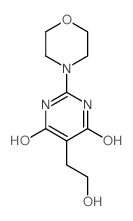
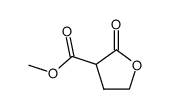
CH5132799
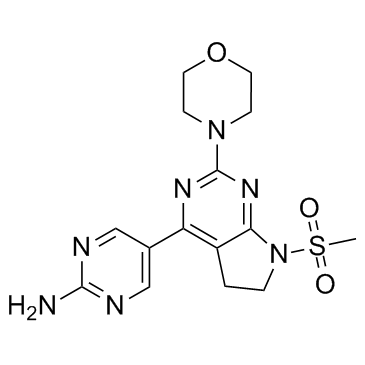
CH5132799 structure
|
Common Name | CH5132799 | ||
|---|---|---|---|---|
| CAS Number | 1007207-67-1 | Molecular Weight | 377.422 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 751.1±70.0 °C at 760 mmHg | |
| Molecular Formula | C15H19N7O3S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 408.1±35.7 °C | |
Use of CH5132799CH5132799 is a selective class I PI3K inhibitor. CH5132799 inhibits class I PI3Ks, particularly PI3Kα, with an IC50 of 14 nM. |
| Name | 5-(7-methylsulfonyl-2-morpholin-4-yl-5,6-dihydropyrrolo[2,3-d]pyrimidin-4-yl)pyrimidin-2-amine |
|---|---|
| Synonym | More Synonyms |
| Description | CH5132799 is a selective class I PI3K inhibitor. CH5132799 inhibits class I PI3Ks, particularly PI3Kα, with an IC50 of 14 nM. |
|---|---|
| Related Catalog | |
| Target |
PI3Kα:14 nM (IC50) PI3Kα-H1047R:5.6 nM (IC50) PI3Kα-E545K:6.7 nM (IC50) PI3Kα-E542K:6.7 nM (IC50) PI3Kγ:36 nM (IC50) PI3Kβ:120 nM (IC50) PI3Kδ:500 nM (IC50) PI3KC2β:5.3 μM (IC50) mTOR:1.6 μM (IC50) |
| In Vitro | CH5132799 is a selective class I PI3K inhibitor with potent antitumor activity against tumors harboring the PIK3CA mutations. CH5132799 selectively inhibits class I PI3Ks and PI3Kα mutants in in vitro kinase assays. CH5132799 inhibits class I PI3Ks, particularly PI3Kα, with an IC50 of 14 nM. IC50 values against class II PI3Ks (C2α and C2β), a class III PI3K (Vps34), and a class IV PI3K (mTOR) are more than 100-fold higher than that against PI3Kα. Interestingly, slightly lower IC50 values are observed against PI3Kα with oncogenic mutations E542K, E545K, and H1047R than against wild-type (WT) PI3Kα. In an analysis of cocrystal structure with PI3Kγ (PBD ID: 3APC), CH5132799 is shown to interact with ATP-binding sites of the enzyme, suggesting an ATP competitive mode of inhibition. No significant inhibitory activities of CH5132799 are observed against a representative panel of 26 protein kinases, including RTKs, nonreceptor tyrosine kinases, and serine/threonine kinases. These data indicate that CH5132799 is a selective class I PI3K inhibitor, especially against PI3Kα and its mutants. CH5132799 shows superior antiproliferative activity across the 4 tumor types, with 75% (45/60) of lines having an IC50 below 1 μM and 38% (23/60) of lines having an IC50 below 0.3 μM[1]. |
| In Vivo | Mice bearing BT-474 tumors (n=14) are orally administered 50 mg/kg of Everolimus on a daily basis for 31 days and then randomized. After randomization, the mice are orally administered 50 mg/kg of Everolimus (n=4) and 12.5 mg/kg (n=5), and 25 mg/kg (n=5) of CH5132799 on a daily basis for 7 days. C, the vehicle-, Everolimus, and CH5132799-treated (25 mg/kg) tumors are resected at 4 hours after terminal administration in B, lysed, and analyzed by Western blotting. CH5132799 administration leads to a remarkable regression in a dose-dependent manner of the tumors regrown after the long-term Everolimus treatment. The tumors are resected at the end of treatment and analyzed by Western blotting with respect to PI3K pathway inhibition. CH5132799 suppresses various effectors in the PI3K pathway, including Akt, FoxO1, S6K, S6, and 4E-BP1, whereas Everolimus inhibits only phosphorylation of S6K and S6, both downstream effectors of mTORC1[1]. |
| Cell Assay | The cell lines are added to the wells of 96-well plates containing 0.076 to 10,000 nM CH5132799 and incubated at 37°C. After 4 days of incubation, Cell Counting Kit-8 solution is added and, after incubation for several more hours, absorbance at 450 nm is measured with Microplate-Reader iMark. The antiproliferative activity is calculated. The IC50 values are calculated[1]. |
| Animal Admin | Mice[1] Female BALB-nu/nu mice (CAnN.Cg-Foxn1/CrlCrlj nu/nu) are used. A total of 4×106 to 1.2×107 cells are suspended in 100 to 200 μL serum-free culture medium and injected subcutaneously into the right flank of the mice. Tumor size is measured by using a gauge twice per week, and tumor volume (TV) is calculated. Once the tumors reach a volume of approximately 200 to 300 mm3, animals are randomized into groups (n=4 or 5 in each group) and treatment is initiated. CH5132799 and Everolimus are orally administered once a day and Trastuzumab is intravenously injected once a week. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 751.1±70.0 °C at 760 mmHg |
| Molecular Formula | C15H19N7O3S |
| Molecular Weight | 377.422 |
| Flash Point | 408.1±35.7 °C |
| Exact Mass | 377.127014 |
| PSA | 136.54000 |
| LogP | -0.87 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.706 |
| Storage condition | -20℃ |
| Hazard Codes | Xi |
|---|
|
~95% 
CH5132799 CAS#:1007207-67-1 |
| Literature: Chugai Seiyaku Kabushiki Kaisha Patent: EP2239261 A1, 2010 ; Location in patent: Page/Page column 38-39 ; |
|
~% 
CH5132799 CAS#:1007207-67-1 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 21, # 6 p. 1767 - 1772 |
|
~% 
CH5132799 CAS#:1007207-67-1 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 21, # 6 p. 1767 - 1772 |
|
~% 
CH5132799 CAS#:1007207-67-1 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 21, # 6 p. 1767 - 1772 |
| cc-614 |
| 2-Pyrimidinamine, 5-[6,7-dihydro-7-(methylsulfonyl)-2-(4-morpholinyl)-5H-pyrrolo[2,3-d]pyrimidin-4-yl]- |
| 5-(7-methanesulfonyl-2-morpholin-4-yl-6,7-dihydro-5H-pyrrolo[2,3-d]pyrimidin-4-yl)-pyrimidin-2-ylamine |
| 5-[7-(Methylsulfonyl)-2-(4-morpholinyl)-6,7-dihydro-5H-pyrrolo[2,3-d]pyrimidin-4-yl]-2-pyrimidinamine |
| 5-(7-(methylsulfonyl)-2-morpholino-6,7-dihydro-5H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrimidin-2-amine |
| QCR-47 |
| CH5132799 |
![4-(4-CHLORO-7-(METHYLSULFONYL)-6,7-DIHYDRO-5H-PYRROLO[2,3-D]PYRIMIDIN-2-YL)MORPHOLINE structure](https://image.chemsrc.com/caspic/169/1178564-27-6.png)