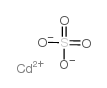
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
EV2700000
-
CAS REGISTRY NUMBER :
-
10124-36-4
-
LAST UPDATED :
-
199712
-
DATA ITEMS CITED :
-
65
-
MOLECULAR FORMULA :
-
O4-S.Cd
-
MOLECULAR WEIGHT :
-
208.46
-
WISWESSER LINE NOTATION :
-
CD S-O4
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
280 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
88 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
6879 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
105 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
27 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Amphibian - frog
-
DOSE/DURATION :
-
105 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
35230 mg/kg
-
SEX/DURATION :
-
female 1-19 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea) Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - maternal-fetal exchange
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
35230 mg/kg
-
SEX/DURATION :
-
female 1-19 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
5150 ug/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - other effects to embryo
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
5150 ug/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
3 mg/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
1030 ug/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
2570 ug/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
28 ug/kg
-
SEX/DURATION :
-
female 12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
2800 ug/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
2 mg/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - Central Nervous System
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
2 mg/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Specific Developmental Abnormalities - homeostasis Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
2 mg/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - eye/ear
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
2 mg/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - homeostasis
-
TYPE OF TEST :
-
Micronucleus test
MUTATION DATA
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TEST SYSTEM :
-
Rodent - hamster Ovary
-
REFERENCE :
-
MUREAV Mutation Research. (Elsevier Science Pub. B.V., POB 211, 1000 AE Amsterdam, Netherlands) V.1- 1964- Volume(issue)/page/year: 265,45,1992 *** REVIEWS *** ACGIH TLV-TWA 0.01 mg(Cd)/m3, inhalable dust DTLVS* The Threshold Limit Values (TLVs) and Biological Exposure Indices (BEIs) booklet issues by American Conference of Governmental Industrial Hygienists (ACGIH), Cincinnati, OH, 1996 Volume(issue)/page/year: TLV/BEI,1997 ACGIH TLV-TWA 0.002 mg(Cd)/m3, respirable dust DTLVS* The Threshold Limit Values (TLVs) and Biological Exposure Indices (BEIs) booklet issues by American Conference of Governmental Industrial Hygienists (ACGIH), Cincinnati, OH, 1996 Volume(issue)/page/year: TLV/BEI,1997 ACGIH TLV-Suspected human carcinogen DTLVS* The Threshold Limit Values (TLVs) and Biological Exposure Indices (BEIs) booklet issues by American Conference of Governmental Industrial Hygienists (ACGIH), Cincinnati, OH, 1996 Volume(issue)/page/year: TLV/BEI,1997 IARC Cancer Review:Animal Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 2,74,1973 IARC Cancer Review:Animal Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 11,39,1976 IARC Cancer Review:Animal Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 58,119,1993 IARC Cancer Review:Human Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 58,119,1993 IARC Cancer Review:Group 1 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 58,119,1993 TOXICOLOGY REVIEW ADTEAS Advances in Teratology. (New York, NY) V.1-5, 1966-72. Discontinued. Volume(issue)/page/year: 5,51,1972 TOXICOLOGY REVIEW FCTXAV Food and Cosmetics Toxicology. (London, UK) V.1-19, 1963-81. For publisher information, see FCTOD7. Volume(issue)/page/year: 9,105,1971 TOXICOLOGY REVIEW PEXTAR Progress in Experimental Tumor Research. (S. Karger AG, Postfach CH-4009 Basel, Switzerland) V.1- 1960- Volume(issue)/page/year: 12,102,1969 TOXICOLOGY REVIEW 43QNAO "Cadmium in the Environment," Nriagu, J.O., ed., 2 vols., New York, John Wiley & Sons, Inc., 1980-81 Volume(issue)/page/year: 2,743,1981 *** U.S. STANDARDS AND REGULATIONS *** MSHA STANDARD-air:TWA 0.2 mg(Cd)/m3 DTLVS* The Threshold Limit Values (TLVs) and Biological Exposure Indices (BEIs) booklet issues by American Conference of Governmental Industrial Hygienists (ACGIH), Cincinnati, OH, 1996 Volume(issue)/page/year: 3,34,1971 *** OCCUPATIONAL EXPOSURE LIMITS *** OEL-ARAB Republic of Egypt:TWA 0.05 mg(Cd)/m3 JAN 1993 OEL-AUSTRALIA:TWA 0.05 mg(Cd)/m3 JAN 1993 OEL-BELGIUM:TWA 0.05 mg(Cd)/m3 JAN 1993 OEL-DENMARK:TWA 0.01 mg(Cd)/m3 JAN 1993 OEL-FINLAND:TWA 0.02 mg(Cd)/m3;Carcinogen JAN 1993 OEL-GERMANY;Carcinogen JAN 1993 OEL-INDIA:TWA 0.05 mg(Cd)/m3 JAN 1993 OEL-JAPAN:TWA 0.05 mg(Cd)/m3 JAN 1993 OEL-THE NETHERLANDS:TWA 0.02 mg(Cd)/m3;STEL 0.1 mg(Cd)/m3 JAN 1993 OEL-THE PHILIPPINES:TWA 0.2 mg(Cd)/m3 JAN 1993 OEL-RUSSIA:TWA 0.01 mg(Cd)/m3;STEL 0.05 mg(Cd)/m3 JAN 1993 OEL-SWEDEN:TWA 0.02 mg(Cd)/m3;Carcinogen JAN 1993 OEL-SWITZERLAND:TWA 0.05 mg(Cd)/m3 JAN 1993 OEL-THAILAND:TWA 0.2 mg(Cd)/m3;STEL 0.5 mg(Cd)/m3 JAN 1993 OEL-TURKEY:TWA 0.2 mg(Cd)/m3 JAN 1993 OEL-UNITED KINGDOM:TWA 0.01 mg(Cd)/m3 JAN 1993 OEL IN BULGARIA, COLOMBIA, JORDAN, KOREA check ACGIH TLV OEL IN NEW ZEALAND, SINGAPORE, VIETNAM check ACGIH TLV *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH RECOMMENDED EXPOSURE LEVEL (REL) : NIOSH REL TO CADMIUM, dust and fume-air:CA lowest feasible conc. REFERENCE : NIOSH* National Institute for Occupational Safety and Health, U.S. Dept. of Health, Education, and Welfare, Reports and Memoranda. Volume(issue)/page/year: DHHS #92-100,1992 NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOHS - National Occupational Hazard Survey (1974) NOHS Hazard Code - 80247 No. of Facilities: 1041 (estimated) No. of Industries: 5 No. of Occupations: 5 No. of Employees: 15328 (estimated) NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - 80247 No. of Facilities: 79 (estimated) No. of Industries: 5 No. of Occupations: 4 No. of Employees: 1313 (estimated) No. of Female Employees: 172 (estimated)
|