cadmium nitrate
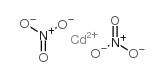
cadmium nitrate structure
|
Common Name | cadmium nitrate | ||
|---|---|---|---|---|
| CAS Number | 10325-94-7 | Molecular Weight | 236.42100 | |
| Density | 2.455(17/4℃) | Boiling Point | 132℃ | |
| Molecular Formula | CdN2O6 | Melting Point | 59.4℃ | |
| MSDS | Chinese | Flash Point | N/A | |
| Symbol |


GHS08, GHS09 |
Signal Word | Danger | |
| Name | cadmium nitrate |
|---|---|
| Synonym | More Synonyms |
| Density | 2.455(17/4℃) |
|---|---|
| Boiling Point | 132℃ |
| Melting Point | 59.4℃ |
| Molecular Formula | CdN2O6 |
| Molecular Weight | 236.42100 |
| Exact Mass | 237.87900 |
| PSA | 137.76000 |
| LogP | 0.56570 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS08, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H340-H350-H360FD-H373-H411 |
| Precautionary Statements | P201-P273-P308 + P313-P391-P501 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | O:Oxidizingagent;T:Toxic;N:Dangerousfortheenvironment; |
| Risk Phrases | R45 |
| Safety Phrases | S53-S45-S36-S26-S36/37-S60 |
| RIDADR | UN 3082 9/PG 3 |
| WGK Germany | 3 |
| Packaging Group | II |
| Hazard Class | 6.1(b) |
|
An environmentally benign solvothermal method for the synthesis of nanostructured Cd5(OH)8(NO3)2(H2O)2: templates for the generation of nanoporous CdO materials with photocatalytic properties.
Nanoscale 3(4) , 1887-93, (2011) Using Cd(NO(3))(2)·4H(2)O as a precursor and ethanol/water as the solvent, we synthesized Cd(5)(OH)(8)(NO(3))(2)(H(2)O)(2) nanowires and nanobelts through a simple solvothermal method. Unlike the conv... |
|
|
Crystal structures of triazine-3-thione derivatives by reaction with copper and cobalt salts.
Inorg. Chem. 45(7) , 3103-12, (2006) The reaction of 5-methoxy-5,6-diphenyl-4,5-dihydro-2H-[1,2,4]triazine-3-thione L1H2OCH3 with copper(II) chloride leads to the formation of an organic molecule L2 containing two triazine rings linked b... |
|
|
Bioavailability of cadmium-organic complexes to soil alga--an exception to the free ion model.
J. Agric. Food Chem. 52(12) , 3894-9, (2004) It is generally considered that cadmium bioavailability shows a considerable dependence on chemical speciation of Cd in solution, correlates best with the activity of free metal ion (Cd2+) in solution... |
| Cadmium nitrate |
| EINECS 233-710-6 |
| MFCD00010915 |
| Enriched isotope 111Cd spike |