Manganese(II) nitrate
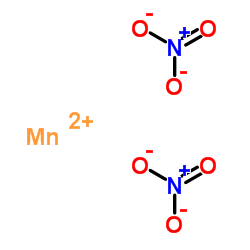
Manganese(II) nitrate structure
|
Common Name | Manganese(II) nitrate | ||
|---|---|---|---|---|
| CAS Number | 10377-66-9 | Molecular Weight | 178.948 | |
| Density | 1.536 g/mL at 25 °C | Boiling Point | 100°C | |
| Molecular Formula | MnN2O6 | Melting Point | 37°C | |
| MSDS | Chinese | Flash Point | N/A | |
| Symbol |


GHS03, GHS06 |
Signal Word | Danger | |
| Name | Manganese nitrate |
|---|---|
| Synonym | More Synonyms |
| Density | 1.536 g/mL at 25 °C |
|---|---|
| Boiling Point | 100°C |
| Melting Point | 37°C |
| Molecular Formula | MnN2O6 |
| Molecular Weight | 178.948 |
| Exact Mass | 178.913681 |
| PSA | 137.76000 |
| LogP | 0.56820 |
| InChIKey | MIVBAHRSNUNMPP-UHFFFAOYSA-N |
| SMILES | O=[N+]([O-])[O-].O=[N+]([O-])[O-].[Mn+2] |
| Water Solubility | soluble |
Synonym: Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: 8 Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
Contact with combustible material may cause fire.The toxicological properties of this material have not been fully investigated. Potential Health Effects Eye: No information regarding eye irritation and other potential effects was found. Skin: No information regarding skin irritation and other potential effects was found. Ingestion: The toxicological properties of this substance have not been fully investigated. Inhalation: The toxicological properties of this substance have not been fully investigated. Chronic: Effects may be delayed. Section 4 - FIRST AID MEASURES Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately. Skin: Get medical aid. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Ingestion: If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately. Inhalation: Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Notes to Physician: Section 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Oxidizer. Greatly increases the burning rate of combustible materials. May accelerate burning if involved in a fire. May explode from heat or contamination. Containers may explode when heated. Extinguishing Media: For small fires, use water spray, dry chemical, carbon dioxide or chemical foam. Cool containers with flooding quantities of water until well after fire is out. For small fires, do NOT use dry chemicals, carbon dioxide, halon or foams. USE WATER ONLY. For large fires flood fire with water from a distance. Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Remove all sources of ignition. Section 7 - HANDLING and STORAGE Handling: Wash thoroughly after handling. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Avoid contact with clothing and other combustible materials. Avoid ingestion and inhalation. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames. Storage: Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Use adequate general or local exhaust ventilation to keep airborne concentrations below the permissible exposure limits. Exposure Limits CAS# 10377-66-9: United Kingdom, WEL - TWA: (listed as manganese, inorganic compounds): 0.5 mg/m3 TWA United Kingdom, WEL - STEL: (listed as manganese, inorganic compounds): 1.5 mg/m3 STEL United States OSHA: 5 mg/m3 Ceiling (as Mn) (listed under Mangan compounds, n.o.s.). Belgium - TWA: (listed as manganese compounds, n.o.s.): 0.2 mg/m3 (as Mn) Japan: (listed as manganese compounds, n.o.s.): 0.3 mg/m3 OEL (ex organic compounds, as Mn) Malaysia: (listed as manganese, inorganic compounds): 0.2 mg/m3 T (as Mn) Netherlands: (listed as manganese compounds, n.o.s.): 3 mg/m3 STE (as Mn) Netherlands: (listed as manganese compounds, n.o.s.): 1 mg/m3 MAC Mn) Spain: (listed as manganese, inorganic compounds): 0.2 mg/m3 VLA- (as Mn) Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Skin: Wear appropriate protective gloves and clothing to prevent skin exposure. Clothing: Wear appropriate protective clothing to minimize contact with skin. Respirators: Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced. Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Solid Color: Not available. Odor: Not available. pH: Not available. Vapor Pressure: Not available. Viscosity: Not available. Boiling Point: Not available. Freezing/Melting Point: Not available. Autoignition Temperature: Not available. Flash Point: Not available. Explosion Limits, lower: Not available. Explosion Limits, upper: Not available. Decomposition Temperature: Solubility in water: Specific Gravity/Density: Molecular Formula: N2O6.Mn Molecular Weight: 124.0098 Section 10 - STABILITY AND REACTIVITY Chemical Stability: Stable under normal temperatures and pressures. Conditions to Avoid: Incompatible materials, combustible materials, organic materials, reducing agents, strong acids. Incompatibilities with Other Materials: Acids (mineral, non-oxidizing, e.g. hydrochloric acid, hydrofluoric acid, muriatic acid, phosphoric acid), acids (organic, e.g. acetic acid, benzoic acid, formic acid, methanoic acid, oxalic acid), alcohols and glycols (e.g. butyl alcohol, ethanol, methanol, ethylene glycol), aldehydes (e.g. acetaldehyde, acrolein, chloral hydrate, formaldehyde), amides (e.g. butyramide, diethyltoluamide, dimethyl formamide), amines (aliphatic and aromatic, e.g. dimethyl amine, propylamine, pyridine, triethylamine), azo, diazo, and hydrazines (e.g. dimethyl hydrazine, hydrazine, methyl hydrazine), carbamates (e.g. carbanolate, carbofuran), cyanides (e.g. potassium cyanide, sodium cyanide), dithiocarbamates (e.g. ferbam, maneb, metham, thiram), esters (e.g. butyl acetate, ethyl acetate, propyl formate), ethers (e.g. dioxane, furfuran, tetrahydrofuran (THF)), hydrocarbons (aromatic, e.g. benzene, chrysene, cumene, toluene), halogenated organics (e.g. dibromoethane, hexachlorobenzene, methyl chloride, trichloroethylene), isocyanates (e.g. methyl isocyanate), ketones (e.g. acetone, acetophenone, MEK, MIBK), mercaptans and other organic sulfides (e.g. butyl mercaptan, carbon disulfide, methanethiol), metals (alkali and alkaline, e.g. cesium, potassium, sodium), metals as powders (e.g. hafnium, raney nickel), metals as non-powders (e.g. brass, bronze, iron), nitrides (e.g. potassium nitride, sodium nitride), nitriles (e.g. acetonitrile, methyl cyanide), nitro compounds (organic, e.g. nitrobenzene, nitroglycerine, picric acid, trinitrotoluene), hydrocarbons (aliphatic, unsaturated, e.g. cyclopentene, ethylene, heptene), hydrocarbons (aliphatic, saturated, e.g. butane, heptane, isooctane), peroxides and hydroperoxides (organic, e.g. acetyl peroxide, benzoyl peroxide, butyl peroxide, methyl ethyl ketone peroxide), phenols and cresols (e.g. carbolic acid, creosote, cresol, phenol, resorcinol), organophosphates, phosphothioates (e.g. methylparathion, parathion, phorate, thionazin), sulfides (i. Hazardous Decomposition Products: Oxides of nitrogen. Hazardous Polymerization: Has not been reported. Section 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 10377-66-9: QU9780000 LD50/LC50: Not available. Carcinogenicity: Manganese(II) Nitrate - Not listed by ACGIH, IARC, or NTP. Other: See actual entry in RTECS for complete information. Section 12 - ECOLOGICAL INFORMATION Section 13 - DISPOSAL CONSIDERATIONS Products which are considered hazardous for supply are classified as Special Waste and the disposal of such chemicals is covered by regulations which may vary according to location. Contact a specialist disposal company or the local waste regulator for advice. Empty containers must be decontaminated before returning for recycling. Section 14 - TRANSPORT INFORMATION IATA Shipping Name: MANGANESE NITRATE Hazard Class: 5.1 UN Number: 2724 Packing Group: III IMO Shipping Name: MANGANESE NITRATE Hazard Class: 5.1 UN Number: 2724 Packing Group: III RID/ADR Shipping Name: MANGANESE NITRATE Hazard Class: 5.1 UN Number: 2724 Packing group: III Section 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: O Risk Phrases: R 8 Contact with combustible material may cause fire. Safety Phrases: WGK (Water Danger/Protection) CAS# 10377-66-9: No information available. Canada CAS# 10377-66-9 is listed on Canada's DSL List. CAS# 10377-66-9 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 10377-66-9 is listed on the TSCA inventory. SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Symbol |


GHS03, GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H272-H315-H319-H331 |
| Precautionary Statements | P220-P261-P305 + P351 + P338-P311 |
| Personal Protective Equipment | Eyeshields;Gloves |
| Hazard Codes | O: Oxidizing agent;C: Corrosive; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S17-S26-S27-S36/37/39-S45-S36 |
| RIDADR | UN 3264 8/PG 3 |
| WGK Germany | 1 |
| Packaging Group | III |
| Hazard Class | 5.1 |
|
Determination of manganese- and manganese-containing fungicides with lucigenin-Tween-20-enhanced chemiluminescence detection.
Luminescence 30 , 950-61, (2015) A flow-injection (FI) method is reported for the determination of Mn(II), maneb and mancozeb fungicides based on the catalytic effect of Mn(II) on the oxidation of lucigenin and dissolved oxygen in a ... |
|
|
Development of optically transparent water oxidation catalysts using manganese pyrophosphate compounds.
J. Photochem. Photobiol. B, Biol. 152 , 139-45, (2015) One challenge in artificial photosynthetic systems is the development of active oxygen evolution catalysts composed of abundant elements. The oxygen evolution activities of manganese pyrophosphate com... |
|
|
MRI phantoms - are there alternatives to agar?
PLoS ONE 8 , e70343, (2013) The suitability of different gelling agents as MRI phantoms was evaluated in terms of homogeneity, gel stability and reproducibility. Time and effort for preparation were also taken into account. The ... |
| manganese dinitrate |
| UNII-I389H78514 |
| Manganese(II) nitrate |
| Manganese(2+) dinitrate |
| EINECS 233-828-8 |
| MFCD00011112 |