LY243246
Modify Date: 2025-11-26 19:14:08
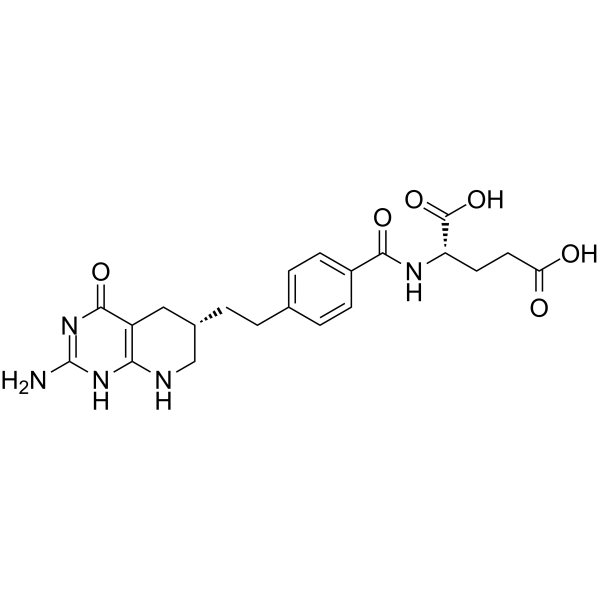
LY243246 structure
|
Common Name | LY243246 | ||
|---|---|---|---|---|
| CAS Number | 106400-18-4 | Molecular Weight | 443.45 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C21H25N5O6 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of LY243246LY243246 ((6S)-DDATHF), the 6S diastereomer of DDATHF, is a potent competitive inhibitor of 5’-phosphoribosylglycinamide formyltransferase (GAR transformylase). 6R- and 6S-diastereomers of DDATHF are remarkably similar and equiactive antimetabolites inhibitory to de novo purine synthesis[1]. |
| Name | LY243246 |
|---|
| Description | LY243246 ((6S)-DDATHF), the 6S diastereomer of DDATHF, is a potent competitive inhibitor of 5’-phosphoribosylglycinamide formyltransferase (GAR transformylase). 6R- and 6S-diastereomers of DDATHF are remarkably similar and equiactive antimetabolites inhibitory to de novo purine synthesis[1]. |
|---|---|
| Related Catalog | |
| In Vitro | LY243246 ((6S)-DDATHF) is a growth inhibitor of mouse L1210 leukemia cells (IC50=29 nM). Both diastereomers of DDATHF are found to be efficient substrates for folylpolyglutamate synthetase of both mouse and human origin (6R- and 6S-diastereomers of DDATHF: Km=7.1 and 9.3 μM for mouse liver folylpolyglutamate synthetase, respectively)[1]. Both the 6R and 6S diastereomers of DDATHF are also cytotoxic to mammalian cells in a stereospecific manner. The cytotoxic potency of (6R)-DDATHF towards different cell lines varied by approximately 14-fold and that of (6S)-DDATHF by as much as 156-fold. Compared to (6R)-DDATHF, (6S)-DDATHF is 6.0- and 7.2-fold more cytotoxic to human WiDr colon adenocarcinoma and Chinese hamster ovary (CHO) cells, respectively, and only 1.5- and 2.0-fold more cytotoxic to human T24 bladder carcinoma and mouse L1210 leukemia cells, respectively. However, compared to (6S)-DDATHF, (6R)-DDATHF was 8.7- and 6.9-fold more cytotoxic to C3H/10T1/2 clone 8 and clone 16 mouse fibroblasts, respectively[2]. |
| References |
| Molecular Formula | C21H25N5O6 |
|---|---|
| Molecular Weight | 443.45 |
| InChIKey | ZUQBAQVRAURMCL-WFASDCNBSA-N |
| SMILES | Nc1nc2c(c(=O)[nH]1)CC(CCc1ccc(C(=O)NC(CCC(=O)O)C(=O)O)cc1)CN2 |
| Hazard Codes | Xi |
|---|