1H-Indole-4-carbaldehyde
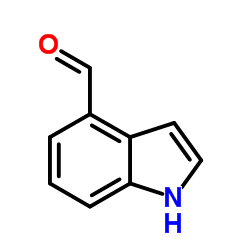
1H-Indole-4-carbaldehyde structure
|
Common Name | 1H-Indole-4-carbaldehyde | ||
|---|---|---|---|---|
| CAS Number | 1074-86-8 | Molecular Weight | 145.16 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 339.1±15.0 °C at 760 mmHg | |
| Molecular Formula | C9H7NO | Melting Point | 139-143 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 166.8±27.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 1H-Indole-4-carbaldehyde1H-Indole-4-carbaldehyde is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | Indole-4-carboxaldehyde |
|---|---|
| Synonym | More Synonyms |
| Description | 1H-Indole-4-carbaldehyde is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog | |
| In Vitro | Indole-4-carboxaldehyde 是一种合成中间体,可用于药物合成。 |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 339.1±15.0 °C at 760 mmHg |
| Melting Point | 139-143 °C(lit.) |
| Molecular Formula | C9H7NO |
| Molecular Weight | 145.16 |
| Flash Point | 166.8±27.8 °C |
| Exact Mass | 145.052765 |
| PSA | 32.86000 |
| LogP | 1.56 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.729 |
| InChIKey | JFDDFGLNZWNJTK-UHFFFAOYSA-N |
| SMILES | O=Cc1cccc2[nH]ccc12 |
| Storage condition | Keep Cold |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H317-H319 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R36;R43 |
| Safety Phrases | S26-S36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
One-pot synthesis of 2-substituted indoles from 2-aminobenzyl phosphonium salts. A formal total synthesis of arcyriacyanin A.
Org. Lett. 10(14) , 3061-3, (2008) The reaction of (2-aminobenzyl) triphenylphosphonium bromide with aromatic aldehydes or alpha,beta-unsaturated aldehydes under microwave-assisted conditions constitutes a new synthesis of 2-substitute... |
|
|
Synthesis and biological evaluation of achiral indole-substituted titanocene dichloride derivatives.
Int J Med Chem 2012 , 905981, (2015) Six new titanocene compounds have been isolated and characterised. These compounds were synthesised from their fulvene precursors using Super Hydride (LiBEt3H) followed by transmetallation with titani... |
|
|
A New Synthesis method of indole-4-carboxaldehyde. Wu Y-M, et al.
Chinese J. Synth. Chem. 12 , 333-35, (2004)
|
| Indole-4-Carboxaldehyde |
| MFCD01632221 |
| 4-Indole-carboxaldehyde |
| 4-Formylindole |
| 1H-Indole-4-carbaldehyde |
| 1H-Indole-4-carboxaldehyde |
| 4-Indolecarbaldehyde |
 CAS#:1074-85-7
CAS#:1074-85-7 CAS#:114615-82-6
CAS#:114615-82-6 CAS#:16136-52-0
CAS#:16136-52-0 CAS#:112447-73-1
CAS#:112447-73-1![3-nitro-2-[2-(1-pyrrolidinyl)ethenyl]benzaldehyde dimethyl acetal Structure](https://image.chemsrc.com/caspic/115/76499-46-2.png) CAS#:76499-46-2
CAS#:76499-46-2 CAS#:39830-66-5
CAS#:39830-66-5 CAS#:3468-22-2
CAS#:3468-22-2 CAS#:59382-59-1
CAS#:59382-59-1![methyl 2-[(E)-2-(dimethylamino)ethenyl]-3-nitrobenzoate Structure](https://image.chemsrc.com/caspic/469/73816-11-2.png) CAS#:73816-11-2
CAS#:73816-11-2 CAS#:83-41-0
CAS#:83-41-0 CAS#:49839-99-8
CAS#:49839-99-8 CAS#:3468-18-6
CAS#:3468-18-6 CAS#:894852-86-9
CAS#:894852-86-9 CAS#:859850-95-6
CAS#:859850-95-6 CAS#:133994-99-7
CAS#:133994-99-7 CAS#:16096-32-5
CAS#:16096-32-5 CAS#:16176-73-1
CAS#:16176-73-1 CAS#:73625-11-3
CAS#:73625-11-3 CAS#:73796-06-2
CAS#:73796-06-2![3-[(dimethylamino)methyl]-1H-indole-4-carbaldehyde structure](https://image.chemsrc.com/caspic/226/74059-91-9.png) CAS#:74059-91-9
CAS#:74059-91-9
