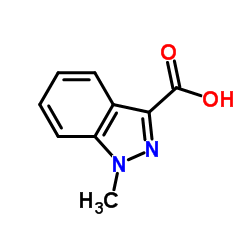
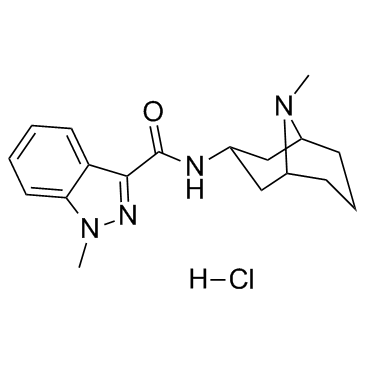
Granisetron

Granisetron structure
|
Common Name | Granisetron | ||
|---|---|---|---|---|
| CAS Number | 109889-09-0 | Molecular Weight | 312.41 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 532.0±40.0 °C at 760 mmHg | |
| Molecular Formula | C18H24N4O | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 275.6±27.3 °C | |
Use of GranisetronGranisetron is a serotonin 5-HT3 receptor antagonist used as an antiemetic to treat nausea and vomiting following chemotherapy.IC50 Value: 17uM (GR reduced 5-HT-evoked contractions) [1]Target: 5-HT3 receptorin vitro: In rat forestomach GR reduced 5-HT-evoked contractions at IC50 17 /- 6 uM. In isolated rabbit heart, GR 0.003-0.03 nM dose-dependently reduced s-HT tachycardia; at high levels GR reduced submaximal and maximal responses to 5-HT [1].in vivo: Leukocyte accumulation was dose-dependently inhibited by granisetron both at 6 and 72 h after induction of inflammation. Granisetron increased PGE(2) level at a lower dose (50 microg/pouch) but higher doses (100 and 200 microg/pouch) inhibited the release. At the same time, TNFalpha production was decreased by the lower dose and increased by higher doses of granisetron in a reciprocal fashion [2]. The GTDS displayed non-inferiority to oral granisetron: complete control was achieved by 60% of patients in the GTDS group, and 65% in the oral granisetron group (treatment difference, -5%; 95% confidence interval, -13-3). Both treatments were well tolerated, the most common adverse event being constipation [3].Clinical trial: Effect of External Heat on a Transdermal Granisetron Patch in Pharmacokinetics (PK) of Healthy Subjects. Phase 1 |
| Name | granisetron |
|---|---|
| Synonym | More Synonyms |
| Description | Granisetron is a serotonin 5-HT3 receptor antagonist used as an antiemetic to treat nausea and vomiting following chemotherapy.IC50 Value: 17uM (GR reduced 5-HT-evoked contractions) [1]Target: 5-HT3 receptorin vitro: In rat forestomach GR reduced 5-HT-evoked contractions at IC50 17 /- 6 uM. In isolated rabbit heart, GR 0.003-0.03 nM dose-dependently reduced s-HT tachycardia; at high levels GR reduced submaximal and maximal responses to 5-HT [1].in vivo: Leukocyte accumulation was dose-dependently inhibited by granisetron both at 6 and 72 h after induction of inflammation. Granisetron increased PGE(2) level at a lower dose (50 microg/pouch) but higher doses (100 and 200 microg/pouch) inhibited the release. At the same time, TNFalpha production was decreased by the lower dose and increased by higher doses of granisetron in a reciprocal fashion [2]. The GTDS displayed non-inferiority to oral granisetron: complete control was achieved by 60% of patients in the GTDS group, and 65% in the oral granisetron group (treatment difference, -5%; 95% confidence interval, -13-3). Both treatments were well tolerated, the most common adverse event being constipation [3].Clinical trial: Effect of External Heat on a Transdermal Granisetron Patch in Pharmacokinetics (PK) of Healthy Subjects. Phase 1 |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 532.0±40.0 °C at 760 mmHg |
| Molecular Formula | C18H24N4O |
| Molecular Weight | 312.41 |
| Flash Point | 275.6±27.3 °C |
| PSA | 50.16000 |
| LogP | 1.47 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.690 |
| Storage condition | -20℃ |
| HS Code | 2934999090 |
|---|
|
~% 
Granisetron CAS#:109889-09-0 |
| Literature: Journal of Medicinal Chemistry, , vol. 33, # 7 p. 1924 - 1929 |
|
~% 
Granisetron CAS#:109889-09-0 |
| Literature: Journal of Medicinal Chemistry, , vol. 33, # 7 p. 1924 - 1929 |
|
~% 
Granisetron CAS#:109889-09-0 |
| Literature: US2008/242696 A1, ; Page/Page column 3 ; |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| 1-(H)Methyl-N-[(3-endo)-9-methyl-9-azabicyclo[3.3.1]non-3-yl]-1H-indazole-3-carboxamide |
| Kevatril |
| BIDD:GT0272 |
| Sancuso |
| [3H]-Granisetron |
| UNII-WZG3J2MCOL |
| 1-methyl-N-[(1S,5R)-9-methyl-9-azabicyclo[3.3.1]nonan-3-yl]indazole-3-carboxamide |
| HMS2089P14 |
| granisetron hydrochloride |
| 1H-Indazole-3-carboxamide, 1-(methyl-d)-N-[(3-endo)-9-methyl-9-azabicyclo[3.3.1]non-3-yl]- |
![exo-3-Amino-9-methyl-9-azabicyclo[3,3,1]nonane structure](https://image.chemsrc.com/caspic/023/76272-41-8.png)