CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
TL2005000
-
CHEMICAL NAME :
-
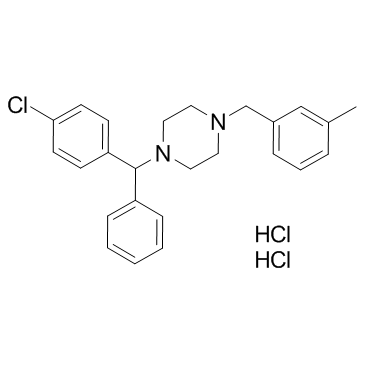
Piperazine, 1-(p-chloro-alpha-phenylbenzyl)-4-(m-methylbenzyl)-, dihydrochloride
-
CAS REGISTRY NUMBER :
-
1104-22-9
-
LAST UPDATED :
-
199803
-
DATA ITEMS CITED :
-
6
-
MOLECULAR FORMULA :
-
C25-H27-Cl-N2.2Cl-H
-
MOLECULAR WEIGHT :
-
463.91
-
WISWESSER LINE NOTATION :
-
T6N DNTJ AYR&R DG& D1R C1 &GH 2
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1600 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
29ZVAB "Handbook of Analytical Toxicology," Sunshine, I., ed., Cleveland, OH, Chemical Rubber Co., 1969 Volume(issue)/page/year: -,67,1969
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
625 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold Behavioral - excitement Lungs, Thorax, or Respiration - respiratory obstruction
-
REFERENCE :
-
JAPMA8 Journal of the American Pharmaceutical Association, Scientific Edition. (Washington, DC) V.29-49, 1940-60. For publisher information, see JPMSAE. Volume(issue)/page/year: 43,653,1954 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
800 mg/kg
-
SEX/DURATION :
-
female 8-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - homeostasis Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
REFERENCE :
-
MPHEAE Medicina et Pharmacologia Experimentalis. (Basel, Switzerland) V.12-17, 1965-67. For publisher information, see PHMGBN. Volume(issue)/page/year: 15,375,1966
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
800 mg/kg
-
SEX/DURATION :
-
female 8-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
MPHEAE Medicina et Pharmacologia Experimentalis. (Basel, Switzerland) V.12-17, 1965-67. For publisher information, see PHMGBN. Volume(issue)/page/year: 15,375,1966
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1 gm/kg
-
SEX/DURATION :
-
female 8-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
REFERENCE :
-
MPHEAE Medicina et Pharmacologia Experimentalis. (Basel, Switzerland) V.12-17, 1965-67. For publisher information, see PHMGBN. Volume(issue)/page/year: 15,375,1966
|