Cefozopran
Modify Date: 2025-08-21 11:27:50
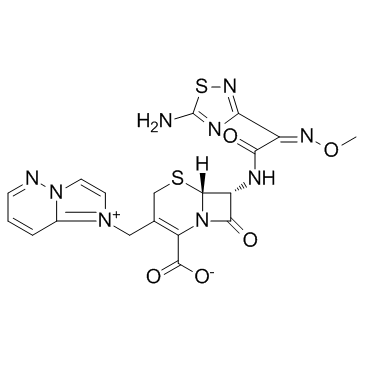
Cefozopran structure
|
Common Name | Cefozopran | ||
|---|---|---|---|---|
| CAS Number | 113359-04-9 | Molecular Weight | 515.526 | |
| Density | 1.59 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C19H17N9O5S2 | Melting Point | >300°C | |
| MSDS | N/A | Flash Point | N/A | |
Use of CefozopranCefozopran(SCE 2787) is a fourth-generation cephalosporin.Target: AntibacterialCefozopran is a fourth-generation cephalosporin. |
| Name | Cefozopran |
|---|---|
| Synonym | More Synonyms |
| Description | Cefozopran(SCE 2787) is a fourth-generation cephalosporin.Target: AntibacterialCefozopran is a fourth-generation cephalosporin. |
|---|---|
| Related Catalog | |
| References |
[1]. http://www.toku-e.com/Assets/MIC/Cefozopran%20hydrochloride.pdf |
| Density | 1.59 g/cm3 |
|---|---|
| Melting Point | >300°C |
| Molecular Formula | C19H17N9O5S2 |
| Molecular Weight | 515.526 |
| Exact Mass | 515.079407 |
| PSA | 238.39000 |
| InChIKey | QDUIJCOKQCCXQY-HEOFFLBTSA-N |
| SMILES | CON=C(C(=O)NC1C(=O)N2C(C(=O)[O-])=C(C[n+]3ccn4ncccc43)CSC12)c1nsc(N)n1 |
| Storage condition | 2-8℃ |
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S24/25 |
| WGK Germany | 2 |
| RTECS | YK0493000 |
| HS Code | 29021990 |
| HS Code | 29021990 |
|---|
| (6R,7R)-7-{[(2Z)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-(methoxyimino)acetyl]amino}-3-(imidazo[1,2-b]pyridazin-1-ium-1-ylmethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate |
| MFCD00883679 |
| Firstcin) |
| sce2787 |
| [6R-[6a,7b(Z)]]-1-[[7-[[(5-Amino-1,2,4-thiadiazol-3-yl)(methoxyimino)acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]imidazo[1,2-b]pyridazinium inner salt |
| Cefozopran |
| Firstcin |
| Firstci |
| Imidazo[1,2-b]pyridazinium, 1-[[(6R,7R)-7-[[(2Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-(methoxyimino)-1-oxoethyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-, inner salt |
| SCE-1787 |