N-Hydroxypipecolic acid
Modify Date: 2024-01-07 21:29:29

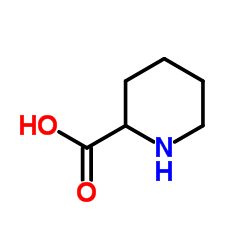
N-Hydroxypipecolic acid structure
|
Common Name | N-Hydroxypipecolic acid | ||
|---|---|---|---|---|
| CAS Number | 115819-92-6 | Molecular Weight | 145.15600 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C6H11NO3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of N-Hydroxypipecolic acidN-Hydroxypipecolic acid (1-Hydroxy-2-piperidinecarboxylic acid), a plant metabolite and a systemic acquired resistance (SAR) regulator, orchestrates SAR establishment in concert with the immune signal salicylic acid. N-Hydroxypipecolic acid accumulates systemically in the plant foliage in response to pathogen attack. N-Hydroxypipecolic acid induces SAR to bacterial and oomycete infection[1][2][3]. |
| Name | 2-Piperidinecarboxylicacid,1-hydroxy-(9CI) |
|---|---|
| Synonym | More Synonyms |
| Description | N-Hydroxypipecolic acid (1-Hydroxy-2-piperidinecarboxylic acid), a plant metabolite and a systemic acquired resistance (SAR) regulator, orchestrates SAR establishment in concert with the immune signal salicylic acid. N-Hydroxypipecolic acid accumulates systemically in the plant foliage in response to pathogen attack. N-Hydroxypipecolic acid induces SAR to bacterial and oomycete infection[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | The mode of action of N-Hydroxypipecolic acid (NHP) in SAR involves direct induction of SAR gene expression, signal amplification, priming for enhanced defense activation and positive interplay with salicylic acid signaling to ensure elevated plant immunity. Flavin-dependent-monooxygenase1 (FMO1) functions downstream of Pip by hydroxylating Pip to generate NHP[1][3]. |
| References |
| Molecular Formula | C6H11NO3 |
|---|---|
| Molecular Weight | 145.15600 |
| Exact Mass | 145.07400 |
| PSA | 60.77000 |
| LogP | 0.25260 |
|
~86% 
N-Hydroxypipeco... CAS#:115819-92-6 |
| Literature: Murahashi, Shun-Ichi; Shiota, Tatsuki Tetrahedron Letters, 1987 , vol. 28, # 51 p. 6469 - 6472 |
|
~% 
N-Hydroxypipeco... CAS#:115819-92-6 |
| Literature: Murahashi, Shun-Ichi; Shiota, Tatsuki Tetrahedron Letters, 1987 , vol. 28, # 51 p. 6469 - 6472 |
| Precursor 1 | |
|---|---|
| DownStream 1 | |
| 3,6-EPOXY-N-HYDROXY-1,2,3,6-TETRAHYDROPHTHALIMIDE |
| N-Hydroxy-DL-pipecolinsaeure |
| N-Hydroxy-1,4-epoxycyclohex-5-en-2,3-dicarboximid |
| 2-hydroxy-3a,4,7,7a-tetrahydro-4,7-epioxido-isoindole-1,3-dione |
| N-Hydroxy-3,6-epoxy-1,2,3,6-tetrahydrophthalimide |
| 1-hydroxy-piperidine-2-carboxylic acid |
| N-Hydroxy-1,4-epoxy-5-cyclohexene-2,3-dicarboximide |
| 3,6-Epoxy-N-hydroxy |


 CAS#:4043-87-2
CAS#:4043-87-2