Pseudouridine 5'-triphosphate
Modify Date: 2025-08-25 17:57:13
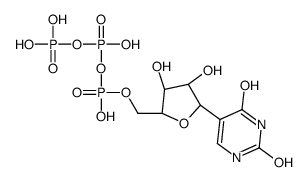
Pseudouridine 5'-triphosphate structure
|
Common Name | Pseudouridine 5'-triphosphate | ||
|---|---|---|---|---|
| CAS Number | 1175-34-4 | Molecular Weight | 484.14100 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C9H15N2O15P3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Pseudouridine 5'-triphosphatepseudouridine-5’-triphosphate (Pseudo-UTP) is one of the most commonly used modified nucleoside for the polymerase-mediated synthesis of RNA molecules. Compared with uridine-containing unmodified mRNAs, the application of pseudouridine-containing modified mRNAs exhibits better nuclease stability, immunogenicity, and translational properties. |
| Name | (1S)-1,4-Anhydro-1-(2,4-dioxo-1,2,3,4-tetrahydro-5-pyrimidinyl)-5 -O-(hydroxy{[hydroxy(phosphonooxy)phosphoryl]oxy}phosphoryl)-D-ri bitol |
|---|---|
| Synonym | More Synonyms |
| Description | pseudouridine-5’-triphosphate (Pseudo-UTP) is one of the most commonly used modified nucleoside for the polymerase-mediated synthesis of RNA molecules. Compared with uridine-containing unmodified mRNAs, the application of pseudouridine-containing modified mRNAs exhibits better nuclease stability, immunogenicity, and translational properties. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C9H15N2O15P3 |
|---|---|
| Molecular Weight | 484.14100 |
| Exact Mass | 483.96900 |
| PSA | 305.18000 |
| Storage condition | -20°C |
| Pseudouridine 5`-Triphosphate |