Fumitremorgin C
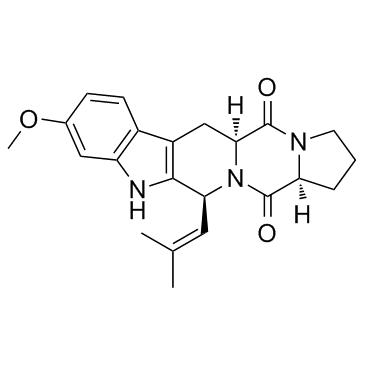
Fumitremorgin C structure
|
Common Name | Fumitremorgin C | ||
|---|---|---|---|---|
| CAS Number | 118974-02-0 | Molecular Weight | 379.452 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 642.9±55.0 °C at 760 mmHg | |
| Molecular Formula | C22H25N3O3 | Melting Point | 259.5-260.5℃ | |
| MSDS | Chinese USA | Flash Point | 342.6±31.5 °C | |
Use of Fumitremorgin CFumitremorgin C is a potent and selective ABCG2/BRCP inhibitor. |
| Name | fumitremorgin C |
|---|---|
| Synonym | More Synonyms |
| Description | Fumitremorgin C is a potent and selective ABCG2/BRCP inhibitor. |
|---|---|
| Related Catalog | |
| In Vitro | Multidrug resistance (MDR) is a major problem in cancer chemotherapy. Fumitremorgin C is extremely effective in reversing resistance to mitoxantrone, doxorubicin, and topotecan in multidrug-selected cell lines. In MCF-7/mtxR (a mitoxantroneselected cell line), fumitremorgin C reverses mitoxantrone resistance (114-fold) and doxorubicin resistance (3-fold). Fumitremorgin C (5/AM)significantly potentiates the toxicity of mitoxantrone (93-fold), doxorubicin (26-fold), and topotecan (24-fold) in S1M1-3.2 cells. Reversal of resistance is associated with an increase in drug accumulation. Fumitremorgin C does not reverse drug resistance in cells with elevated expression of Pgp or MRP[1]. Fumitremorgin C almost completely reverses resistance mediated by BCRP in vitro and is a pharmacological probe for the expression and molecular action of this transporter. Fumitremorgin C also enhances the toxicity of mitoxantrone and topotecan in vector-transfected MCF-7 cells (2.5–5.6 fold). It reduces the IC50 of topotecan in BCRP-overexpressing cells below that observed in the untreated vector-transfected cells. [2]. |
| Cell Assay | Cells are treated with chemotherapeutic agent and the reversal agents Fumitremorgin C is added to cells (0.1 to 80 /UM). In parallel wells, cells are grown in the presence of the reversal agent alone. Following a 3-day growth period, cells are fixed in 10% trichloroacetic acid for 1 h and washed extensively with water, and cell-associated protein is stained using 0.1% SRB. Excess reagent is removed by washing plates in 5% acetic acid, the dye is solubilized in 10 mM Tris base, and absorbance is determined in a UV Max spectrophotometer at 540 nm. Cell survival is determined relative to control wells[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 642.9±55.0 °C at 760 mmHg |
| Melting Point | 259.5-260.5℃ |
| Molecular Formula | C22H25N3O3 |
| Molecular Weight | 379.452 |
| Flash Point | 342.6±31.5 °C |
| Exact Mass | 379.189606 |
| PSA | 65.64000 |
| LogP | 1.74 |
| Appearance of Characters | solid |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.676 |
| InChIKey | DBEYVIGIPJSTOR-FHWLQOOXSA-N |
| SMILES | COc1ccc2c3c([nH]c2c1)C(C=C(C)C)N1C(=O)C2CCCN2C(=O)C1C3 |
| Storage condition | 2-8°C |
| Water Solubility | acetonitrile: 5 mg/mL, soluble |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
The impact of CLAUDIN-1 on follicular thyroid carcinoma aggressiveness.
Endocr. Relat. Cancer 22 , 819-30, (2015) CLAUDIN-1 belongs to the family of transmembrane tight junction proteins tightening the paracellular cleft of epithelial cells. In human malignancies, CLAUDIN-1 is often dysregulated and located in su... |
|
|
Development and validation of an ultra-high performance LC-MS/MS assay for intracellular SN-38 in human solid tumour cell lines: comparison with a validated HPLC-fluorescence method.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 969 , 213-8, (2014) A simple and rapid ultra-high performance liquid chromatography-mass spectrometry/mass spectrometry (UPLC-MS/MS) method has been developed for measuring intracellular concentrations of the anticancer ... |
|
|
Quantitative mass spectrometry imaging of emtricitabine in cervical tissue model using infrared matrix-assisted laser desorption electrospray ionization.
Anal. Bioanal. Chem 407(8) , 2073-84, (2015) A quantitative mass spectrometry imaging (QMSI) technique using infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) is demonstrated for the antiretroviral (ARV) drug emtrici... |
| fumitremorogin C |
| (5aS,12S,14aS)-1,2,5a,6,11,12,14a-octahydro-9-methoxy-12-(2-methylprop-1-en-1-yl)-5H,14H-pyrrolo[1'',4'':4',5']pyrazino[1',2':1,6]pyrido[3,4-b]indole-5,14-dione |
| MFCD08702652 |
| fumitremogin C |
| 5H,14H-Pyrrolo[1'',2'':4',5']pyrazino[1',2':1,6]pyrido[3,4-b]indole-5,14-dione, 1,2,3,5a,6,11,12,14a-octahydro-9-methoxy-12-(2-methyl-1-propen-1-yl)-, (5aS,12S,14aS)- |
| (5aS,12S,14aS)-9-methoxy-12-(2-methylprop-1-en-1-yl)-1,2,3,5a,6,11,12,14a-octahydro-5H,14H-pyrrolo[1'',2'':4',5']pyrazino[1',2':1,6]pyrido[3,4-b]indole-5,14-dione |
| Fumitremorgin C |
| (5aS,12S,14aS)-9-Methoxy-12-(2-methyl-1-propen-1-yl)-1,2,3,5a,6,11,12,14a-octahydro-5H,14H-pyrrolo[1'',2'':4',5']pyrazino[1',2':1,6]pyrido[3,4-b]indole-5,14-dione |

