n-methylphenothiazine
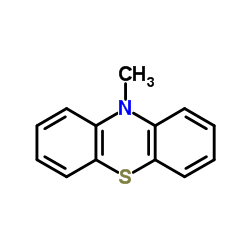
n-methylphenothiazine structure
|
Common Name | n-methylphenothiazine | ||
|---|---|---|---|---|
| CAS Number | 1207-72-3 | Molecular Weight | 213.298 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 341.6±12.0 °C at 760 mmHg | |
| Molecular Formula | C13H11NS | Melting Point | 99-101 °C(lit.) | |
| MSDS | USA | Flash Point | 160.4±19.6 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 10-methylphenothiazine |
|---|---|
| Synonym | More Synonyms |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 341.6±12.0 °C at 760 mmHg |
| Melting Point | 99-101 °C(lit.) |
| Molecular Formula | C13H11NS |
| Molecular Weight | 213.298 |
| Flash Point | 160.4±19.6 °C |
| Exact Mass | 213.061218 |
| PSA | 28.54000 |
| LogP | 4.49 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.665 |
| InChIKey | QXBUYALKJGBACG-UHFFFAOYSA-N |
| SMILES | CN1c2ccccc2Sc2ccccc21 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2934300000 |
| Precursor 9 | |
|---|---|
| DownStream 9 | |
| HS Code | 2934300000 |
|---|---|
| Summary | 2934300000. other compounds containing in the structure a phenothiazine ring-system (whether or not hydrogenated), not further fused. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
[Electrocatalytic oxidation of glutathione at 10-methylphenothiazine modified carbon paste electrode and its practical analytical application].
Yao Xue Xue Bao 43(3) , 291-4, (2008) The electrocatalytic oxidation of glutathione (reduced form GSH) at 10-methylphenothiazine (MPT) modified carbon paste electrode (MPT/CPE) was investigated by cyclic voltammetry (CV). Although GSH its... |
|
|
Phenothiazine as a redox-active DNA base substitute: comparison with phenothiazine-modified uridine.
Org. Biomol. Chem. 6(1) , 48-50, (2008) Phenothiazine can be incorporated as a redox-active probe into DNA in two conceptually different ways: the non-nucleosidic DNA base surrogate exhibits similar properties to 10-methylphenothiazine but ... |
|
|
Incorporation of sulphonated cyclodextrins into polypyrrole: an approach for the electro-controlled delivering of neutral drugs.
Biosens. Bioelectron. 10(1-2) , 219-29, (1995) The electro-controlled delivery of drugs based on the doping-dedoping mechanism of Electro-Conducting Polymers is restricted to charged substances acting as dopants. In order to overcome this limitati... |
| 10-methyl phenothiazine |
| Phenothiazine, 10-methyl- |
| 10H-Phenothiazine,10-methyl |
| n-methylphenothiazine |
| MFCD00041836 |
| 10-Methyl-10H-phenothiazine |
| 10-methylphenothiazine |
| Phenothiazine,10-methyl |
| 10-methyl-10H-phenothiazole |
| 10H-Phenothiazine, 10-methyl- |
| EINECS 214-896-8 |
 CAS#:92-84-2
CAS#:92-84-2 CAS#:74-88-4
CAS#:74-88-4 CAS#:616-38-6
CAS#:616-38-6 CAS#:77-78-1
CAS#:77-78-1 CAS#:74-83-9
CAS#:74-83-9 CAS#:33224-08-7
CAS#:33224-08-7 CAS#:7647-01-0
CAS#:7647-01-0 CAS#:25244-68-2
CAS#:25244-68-2 CAS#:68825-29-6
CAS#:68825-29-6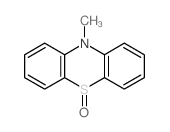 CAS#:2234-09-5
CAS#:2234-09-5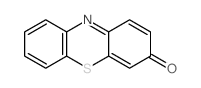 CAS#:581-30-6
CAS#:581-30-6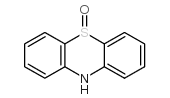 CAS#:1207-71-2
CAS#:1207-71-2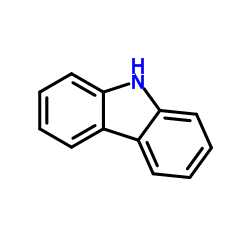 CAS#:86-74-8
CAS#:86-74-8 CAS#:31123-52-1
CAS#:31123-52-1 CAS#:73866-83-8
CAS#:73866-83-8 CAS#:4997-36-8
CAS#:4997-36-8 CAS#:6314-29-0
CAS#:6314-29-0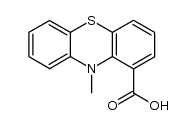 CAS#:107624-59-9
CAS#:107624-59-9
