Ibuprofen-13C6
Modify Date: 2025-08-25 07:02:29
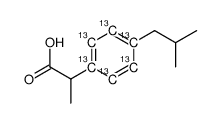
Ibuprofen-13C6 structure
|
Common Name | Ibuprofen-13C6 | ||
|---|---|---|---|---|
| CAS Number | 1216459-54-9 | Molecular Weight | 212.24 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C713C6H18O2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Ibuprofen-13C6Ibuprofen-13C6 ((±)-Ibuprofen-13C6) is a 13C labeled Ibuprofen (HY-78131). Ibuprofen ((±)-Ibuprofen) is a potent, orally active, selective COX-1 inhibitor with an IC50 value of 13 μM. Ibuprofen inhibits cell proliferation, angiogenesis, and induces cell apoptosis. Ibuprofen is a nonsteroidal anti-inflammatory agent and a nitric oxide (NO) donor. Ibuprofen ((±)-Ibuprofen) can be used in the research of pain, swelling, inflammation, infection, immunology, cancers[1][2][3][4][5]. |
| Name | 2-[4-(2-methylpropyl)phenyl]propanoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Ibuprofen-13C6 ((±)-Ibuprofen-13C6) is a 13C labeled Ibuprofen (HY-78131). Ibuprofen ((±)-Ibuprofen) is a potent, orally active, selective COX-1 inhibitor with an IC50 value of 13 μM. Ibuprofen inhibits cell proliferation, angiogenesis, and induces cell apoptosis. Ibuprofen is a nonsteroidal anti-inflammatory agent and a nitric oxide (NO) donor. Ibuprofen ((±)-Ibuprofen) can be used in the research of pain, swelling, inflammation, infection, immunology, cancers[1][2][3][4][5]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C713C6H18O2 |
|---|---|
| Molecular Weight | 212.24 |
| Exact Mass | 212.15100 |
| PSA | 37.30000 |
| LogP | 3.07320 |
| InChIKey | HEFNNWSXXWATRW-HNHUTNRMSA-N |
| SMILES | CC(C)Cc1ccc(C(C)C(=O)O)cc1 |
| 2-(4-Isobutylphenyl)propionic Acid-13C6 |
| Ibuprofen-(ring-13C6) |
| Ibuprofen-13C6 |
| [13C6]-Ibuprofen |