CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
LQ3000000
-
CHEMICAL NAME :
-
Formamide, N-methyl-
-
CAS REGISTRY NUMBER :
-
123-39-7
-
BEILSTEIN REFERENCE NO. :
-
1098352
-
LAST UPDATED :
-
199712
-
DATA ITEMS CITED :
-
40
-
MOLECULAR FORMULA :
-
C2-H5-N-O
-
MOLECULAR WEIGHT :
-
59.08
-
WISWESSER LINE NOTATION :
-
VHM1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
Standard Draize test
-
ROUTE OF EXPOSURE :
-
Administration into the eye
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 39,129,1977 ** ACUTE TOXICITY DATA **
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JRPFA4 Journal of Reproduction and Fertility. (Biochemical Soc. Book Depot, POB 32, Commerce Way, Colchester, Essex CO2 8HP, UK) V.1- 1960- Volume(issue)/page/year: 4,219,1962
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3500 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JRPFA4 Journal of Reproduction and Fertility. (Biochemical Soc. Book Depot, POB 32, Commerce Way, Colchester, Essex CO2 8HP, UK) V.1- 1960- Volume(issue)/page/year: 4,219,1962
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>2500 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
APEPA2 Naunyn-Schmiedebergs Archiv fuer Pharmakologie und Experimentelle Pathologie. (Berlin, Ger.) V.254-263, 1966-69. For publisher information, see NSAPCC. Volume(issue)/page/year: 257,296,1967
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2400 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - corneal damage Behavioral - somnolence (general depressed activity) Musculoskeletal - other changes
-
REFERENCE :
-
GISAAA Gigiena i Sanitariya. For English translation, see HYSAAV. (V/O Mezhdunarodnaya Kniga, 113095 Moscow, USSR) V.1- 1936- Volume(issue)/page/year: 57(2),30,1992
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2600 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
TXCYAC Toxicology. (Elsevier Scientific Pub. Ireland, Ltd., POB 85, Limerick, Ireland) V.1- 1973- Volume(issue)/page/year: 34,173,1985
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
802 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NCISP* National Cancer Institute Screening Program Data Summary, Developmental Therapeutics Program. (Bethesda, MD 20205) Volume(issue)/page/year: JAN1986
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
3100 mg/kg
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - respiratory depression Nutritional and Gross Metabolic - weight loss or decreased weight gain Nutritional and Gross Metabolic - body temperature decrease
-
REFERENCE :
-
DABBBA Dissertation Abstracts International, B: The Sciences and Engineering. (University Microfilms International, 300 N. Zeeb Rd., Ann Arbor, MI 48106) V.30- 1969- Volume(issue)/page/year: 40,549,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1580 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
TXCYAC Toxicology. (Elsevier Scientific Pub. Ireland, Ltd., POB 85, Limerick, Ireland) V.1- 1973- Volume(issue)/page/year: 34,173,1985
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2700 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
TXCYAC Toxicology. (Elsevier Scientific Pub. Ireland, Ltd., POB 85, Limerick, Ireland) V.1- 1973- Volume(issue)/page/year: 34,173,1985
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1175 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - corneal damage Behavioral - somnolence (general depressed activity) Musculoskeletal - other changes
-
REFERENCE :
-
GISAAA Gigiena i Sanitariya. For English translation, see HYSAAV. (V/O Mezhdunarodnaya Kniga, 113095 Moscow, USSR) V.1- 1936- Volume(issue)/page/year: 57(2),30,1992
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
>568 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - food intake (animal) Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
NTIS** National Technical Information Service. (Springfield, VA 22161) Formerly U.S. Clearinghouse for Scientific & Technical Information. Volume(issue)/page/year: PB82-232158
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
465 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - corneal damage Behavioral - somnolence (general depressed activity) Musculoskeletal - other changes
-
REFERENCE :
-
GISAAA Gigiena i Sanitariya. For English translation, see HYSAAV. (V/O Mezhdunarodnaya Kniga, 113095 Moscow, USSR) V.1- 1936- Volume(issue)/page/year: 57(2),30,1992 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
5460 ug/kg/26W-I
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - changes in lung weight Blood - changes in leukocyte (WBC) count Nutritional and Gross Metabolic - changes in chlorine
-
REFERENCE :
-
GISAAA Gigiena i Sanitariya. For English translation, see HYSAAV. (V/O Mezhdunarodnaya Kniga, 113095 Moscow, USSR) V.1- 1936- Volume(issue)/page/year: 57(2),30,1992
-
TYPE OF TEST :
-
TCLo - Lowest published toxic concentration
-
ROUTE OF EXPOSURE :
-
Inhalation
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
400 ppm/6H/2W-I
-
TOXIC EFFECTS :
-
Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol) Nutritional and Gross Metabolic - weight loss or decreased weight gain Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases
-
REFERENCE :
-
FAATDF Fundamental and Applied Toxicology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1981- Volume(issue)/page/year: 14,810,1990
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
5603 mg/kg/5D-I
-
TOXIC EFFECTS :
-
Liver - hepatitis (hepatocellular necrosis), zonal Endocrine - other changes Related to Chronic Data - death
-
REFERENCE :
-
NTIS** National Technical Information Service. (Springfield, VA 22161) Formerly U.S. Clearinghouse for Scientific & Technical Information. Volume(issue)/page/year: PB82-232158
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
4 gm/kg/10D-I
-
TOXIC EFFECTS :
-
Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - dehydrogenases Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases
-
REFERENCE :
-
TXCYAC Toxicology. (Elsevier Scientific Pub. Ireland, Ltd., POB 85, Limerick, Ireland) V.1- 1973- Volume(issue)/page/year: 34,173,1985
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
662 mg/kg/5D-I
-
TOXIC EFFECTS :
-
Liver - hepatitis (hepatocellular necrosis), zonal Endocrine - other changes Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - phosphatases
-
REFERENCE :
-
NTIS** National Technical Information Service. (Springfield, VA 22161) Formerly U.S. Clearinghouse for Scientific & Technical Information. Volume(issue)/page/year: PB82-232158 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1 gm/kg
-
SEX/DURATION :
-
female 7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - homeostasis Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
REFERENCE :
-
85DJA5 "Malformations Congenitales des Mammiferes," Tuchmann-Duplessis, H., Paris, Masson et Cie, 1971 Volume(issue)/page/year: -,95,1971
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
750 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
FAATDF Fundamental and Applied Toxicology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1981- Volume(issue)/page/year: 27,239,1995
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
750 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
FAATDF Fundamental and Applied Toxicology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1981- Volume(issue)/page/year: 27,239,1995
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
DOSE :
-
1200 mg/kg
-
SEX/DURATION :
-
female 12-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - body wall
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 41,35,1977
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
DOSE :
-
400 mg/kg
-
SEX/DURATION :
-
female 12-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 41,35,1977
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
DOSE :
-
600 mg/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 41,35,1977
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
700 mg/kg
-
SEX/DURATION :
-
female 7-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
REFERENCE :
-
85DJA5 "Malformations Congenitales des Mammiferes," Tuchmann-Duplessis, H., Paris, Masson et Cie, 1971 Volume(issue)/page/year: -,95,1971
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
500 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
REFERENCE :
-
APEPA2 Naunyn-Schmiedebergs Archiv fuer Pharmakologie und Experimentelle Pathologie. (Berlin, Ger.) V.254-263, 1966-69. For publisher information, see NSAPCC. Volume(issue)/page/year: 257,296,1967
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
200 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 17,610,1967
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
400 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 17,610,1967
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
DOSE :
-
400 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 17,610,1967
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
DOSE :
-
800 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 17,610,1967
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
70 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
REFERENCE :
-
DABBBA Dissertation Abstracts International, B: The Sciences and Engineering. (University Microfilms International, 300 N. Zeeb Rd., Ann Arbor, MI 48106) V.30- 1969- Volume(issue)/page/year: 40,549,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
650 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 30,1557,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
650 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 30,1557,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
650 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - Central Nervous System
-
REFERENCE :
-
FAATDF Fundamental and Applied Toxicology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1981- Volume(issue)/page/year: 27,239,1995
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
650 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Specific Developmental Abnormalities - gastrointestinal system
-
REFERENCE :
-
FAATDF Fundamental and Applied Toxicology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1981- Volume(issue)/page/year: 27,239,1995
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
DOSE :
-
1800 mg/kg
-
SEX/DURATION :
-
female 8-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 41,35,1977
|




 CAS#:107-31-3
CAS#:107-31-3 CAS#:74-89-5
CAS#:74-89-5 CAS#:10352-28-0
CAS#:10352-28-0 CAS#:74-88-4
CAS#:74-88-4 CAS#:18952-41-5
CAS#:18952-41-5 CAS#:201230-82-2
CAS#:201230-82-2 CAS#:593-51-1
CAS#:593-51-1 CAS#:64-18-6
CAS#:64-18-6 CAS#:75-50-3
CAS#:75-50-3 CAS#:50-00-0
CAS#:50-00-0 CAS#:1117-72-2
CAS#:1117-72-2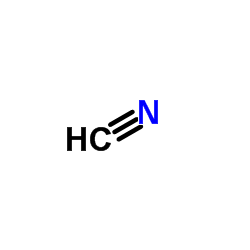 CAS#:74-90-8
CAS#:74-90-8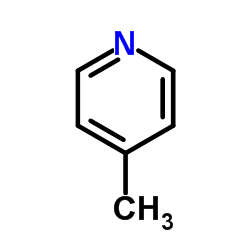 CAS#:108-89-4
CAS#:108-89-4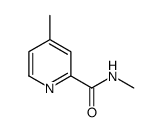 CAS#:107427-71-4
CAS#:107427-71-4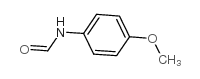 CAS#:5470-34-8
CAS#:5470-34-8 CAS#:3717-38-2
CAS#:3717-38-2 CAS#:937602-15-8
CAS#:937602-15-8 CAS#:4597-87-9
CAS#:4597-87-9