Icariside E5
Modify Date: 2024-01-03 18:33:07
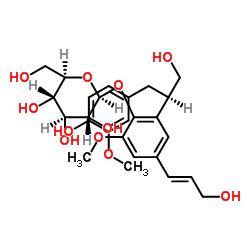
Icariside E5 structure
|
Common Name | Icariside E5 | ||
|---|---|---|---|---|
| CAS Number | 126176-79-2 | Molecular Weight | 522.542 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 807.6±65.0 °C at 760 mmHg | |
| Molecular Formula | C26H34O11 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 442.2±34.3 °C | |
Use of Icariside E5Icariside E5 is a lignan glycoside isolated from the Albiziae Cortex. Icariside E5 promotes the proliferation of HUVECs without cytotoxicity. Icariside E5 has antioxidant properties[1][2]. |
| Name | 2-[(2R)-1-Hydroxy-3-(4-hydroxy-3-methoxyphenyl)-2-propanyl]-4-[(1 E)-3-hydroxy-1-propen-1-yl]-6-methoxyphenyl β-D-glucopyranoside |
|---|---|
| Synonym | More Synonyms |
| Description | Icariside E5 is a lignan glycoside isolated from the Albiziae Cortex. Icariside E5 promotes the proliferation of HUVECs without cytotoxicity. Icariside E5 has antioxidant properties[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Icariside E5 (5, 10, 20, 40 µM; for 48 h) slightly promotes the proliferation of human umbilical vein endothelial cells (HUVECs). Icariside E5 does not have the pharmacological effect of scavenging ROS[1]. Icariside E neither induces an increase in the intracellular levels of reactive oxygen species nor affects the mitochondria permeability transition, and it does not signal through the vanilloid receptor type 1. It protects Jurkat cells from apoptosis induced by the oxidative stress mediated by serum withdrawal[2]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 807.6±65.0 °C at 760 mmHg |
| Molecular Formula | C26H34O11 |
| Molecular Weight | 522.542 |
| Flash Point | 442.2±34.3 °C |
| Exact Mass | 522.210083 |
| PSA | 178.53000 |
| LogP | -1.42 |
| Vapour Pressure | 0.0±3.0 mmHg at 25°C |
| Index of Refraction | 1.650 |
| Hazard Codes | Xi |
|---|
| β-D-Glucopyranoside, 2-[(1R)-2-hydroxy-1-[(4-hydroxy-3-methoxyphenyl)methyl]ethyl]-4-[(1E)-3-hydroxy-1-propen-1-yl]-6-methoxyphenyl |
| icariside E5 |
| Icariside E5 |

