Ciclesonide
Modify Date: 2024-01-02 20:20:19
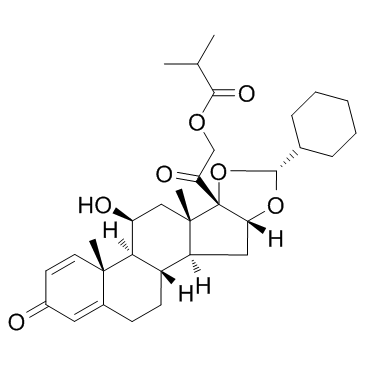
Ciclesonide structure
|
Common Name | Ciclesonide | ||
|---|---|---|---|---|
| CAS Number | 126544-47-6 | Molecular Weight | 540.68800 | |
| Density | 1.23 g/cm3 | Boiling Point | 665ºC at 760 mmHg | |
| Molecular Formula | C32H44O7 | Melting Point | 202-209?C | |
| MSDS | N/A | Flash Point | 210ºC | |
Use of CiclesonideCiclesonide(RPR251526) is a glucocorticoid used to treat obstructive airway diseases.Target: Glucocorticoid ReceptorCiclesonide (CIC) is an inhaled glucocorticosteroid. CIC (5 microM) was rapidly hydrolyzed by NHBE cells (approximately 30% conversion at 4h), with almost complete conversion by 24h. In liver and NHBE cells, major involvement of cytosolic carboxylesterases, with some contribution by cholinesterases, was indicated. The highest level of conversion was found in the liver, the site of inactivation of des-CIC through rapid oxidation by cytochrome P450. Carboxylesterases in bronchial epithelial cells probably contribute significantly to the conversion to des-CIC in the target organ, whereas low systemic levels of des-CIC are a result of the high metabolic clearance by the liver following CIC inhalation [1]. Ciclesonide may have some advantage although it is not as yet licensed in all countries for paediatric use [2]. |
| Name | Ciclesonide |
|---|---|
| Synonym | More Synonyms |
| Description | Ciclesonide(RPR251526) is a glucocorticoid used to treat obstructive airway diseases.Target: Glucocorticoid ReceptorCiclesonide (CIC) is an inhaled glucocorticosteroid. CIC (5 microM) was rapidly hydrolyzed by NHBE cells (approximately 30% conversion at 4h), with almost complete conversion by 24h. In liver and NHBE cells, major involvement of cytosolic carboxylesterases, with some contribution by cholinesterases, was indicated. The highest level of conversion was found in the liver, the site of inactivation of des-CIC through rapid oxidation by cytochrome P450. Carboxylesterases in bronchial epithelial cells probably contribute significantly to the conversion to des-CIC in the target organ, whereas low systemic levels of des-CIC are a result of the high metabolic clearance by the liver following CIC inhalation [1]. Ciclesonide may have some advantage although it is not as yet licensed in all countries for paediatric use [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.23 g/cm3 |
|---|---|
| Boiling Point | 665ºC at 760 mmHg |
| Melting Point | 202-209?C |
| Molecular Formula | C32H44O7 |
| Molecular Weight | 540.68800 |
| Flash Point | 210ºC |
| Exact Mass | 540.30900 |
| PSA | 99.13000 |
| LogP | 4.70390 |
| Vapour Pressure | 1.61E-20mmHg at 25°C |
| Index of Refraction | 1.575 |
| Storage condition | Refrigerator |
| HS Code | 2937229000 |
|---|
| HS Code | 2937229000 |
|---|
| 2-[(4aR,4bS,5S,6aS,6bS,8R,9aR,10aS,10bS)-8-Cyclohexyl-5-hydroxy-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,9a,10,10a,10b,11,12-dodecahydro-6bH-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-6b-yl]-2-oxoethyl-2-methylpropanoat |
| (R)-11b,16a,17,21-Tetrahydroxypregna-1,4-diene-3,20-dione Cyclic 16,17-Acetal with Cyclohexanecarboxaldehyde 21-Isobutyrate |
| 2-méthylpropanoate de 2-[(4aR,4bS,5S,6aS,6bS,8R,9aR,10aS,10bS)-8-cyclohexyl-5-hydroxy-4a,6a-diméthyl-2-oxo-2,4a,4b,5,6,6a,9a,10,10a,10b,11,12-dodécahydro-6bH-naphto[2',1':4,5]indéno[1,2-d][1,3]dioxol-6b-yl]-2-oxoéthyle |
| Propanoic acid, 2-methyl-, 2-[(4aR,4bS,5S,6aS,6bS,8R,9aR,10aS,10bS)-8-cyclohexyl-2,4a,4b,5,6,6a,9a,10,10a,10b,11,12-dodecahydro-5-hydroxy-4a,6a-dimethyl-2-oxo-6bH-naphth[2',1':4,5]indeno[1,2-d][1,3]dioxol-6b-yl]-2-oxoethyl ester |
| 2-[(4aR,4bS,5S,6aS,6bS,8R,9aR,10aS,10bS)-8-Cyclohexyl-5-hydroxy-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,9a,10,10a,10b,11,12-dodecahydro-6bH-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-6b-yl]-2-oxoethyl 2-methylpropanoate |
| 2-méthylpropanoate de 2-[(4aR,4bS,5S,6aS,6bS,8R,9aR,10aS,10bS)-8-cyclohexyl-5-hydroxy-4a,6a-diméthyl-2-oxo-2,4a,4b,5,6,6a,9a,10,10a,10b,11,12-dodécahydro-6bH-naphto[2',1':4,5]indéno[1,2-d][1,3]dioxol- |
| Alvesco |
| Ciclesonide |
| propanoic acid, 2-methyl-, 2-[(4aR,4bS,5S,6aS,6bS,8R,9aR,10aS,10bS)-8-cyclohexyl-2,4a,4b,5,6,6a,9a,10,10a,10b,11,12-dodecahydro-5-hydroxy-4a,6a-dimethyl-2-oxo-6bH-naphth[2',1':4,5]indeno[1,2-d][1,3]di |
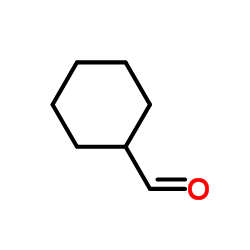 CAS#:2043-61-0
CAS#:2043-61-0 CAS#:996-30-5
CAS#:996-30-5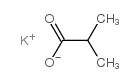 CAS#:19455-20-0
CAS#:19455-20-0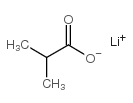 CAS#:25179-23-1
CAS#:25179-23-1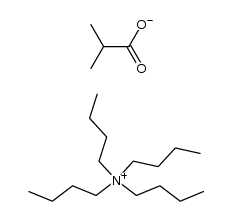 CAS#:60154-69-0
CAS#:60154-69-0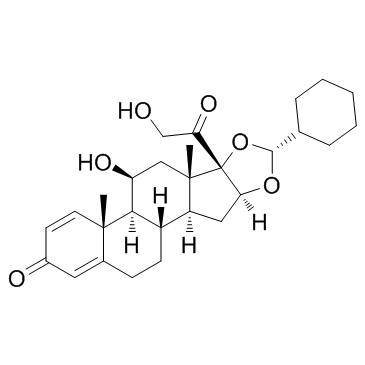 CAS#:161115-59-9
CAS#:161115-59-9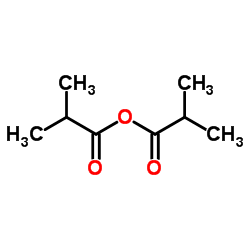 CAS#:97-72-3
CAS#:97-72-3